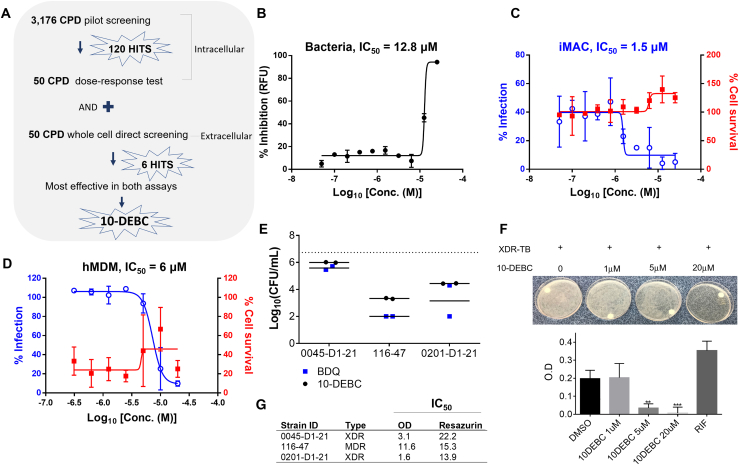
Figure 7.
Anti-TB Activity of 10-DEBC
(A) Schematic illustration of screening assay to narrow down the effective compounds.
(B) Dose-response curve of 10-DEBC against H37Rv-GFP replicating in culture medium. Data represent percent growth inhibition relative to the DMSO-treated control.
(C) Dose-response curve of 10-DEBC against H37Rv-GFP replicating inside iMACs. Data represent percentages of Mtb-infected iMACs (blue line) and surviving iMACs (red line) at 5 days after infection.
(D) Dose-response curve of 10-DEBC against H37Rv-GFP replicating in infected hMDMs treated with 10-DEBC for 5 days.
(E) Anti-mycobacterial activity of 10-DEBC against XDR Mtb replicating inside Raw264.7cells. TB-infected cells were treated with vehicle or 10-DEBC at indicated concentrations for 5 days. Cell lysates were serially diluted and 10 μL of each dilution plated in 7H11 Agar in duplicates. Colonies were counted after 21 days incubation; bedaquiline (BDQ) was used as a positive control. (B–E) Average of two independent experiments.
(F) XDR-TB-infected iMACs were treated with vehicle or 10-DEBC for 5 days. Upper images of intracellular bacterial growth on 7H10 Agar plates. The colonies were suspended in liquid media and the bacterial load was monitored by optical density at 600 nm (OD600) measurement. 10 μM of rifampicin (RIF).
(G) Extracellular anti-mycobacterial activity of 10-DEBC against a panel of three Mtb drug-resistant clinical isolates grown in 7H9 complete medium (~105/well) and treated with test compound for 5 days. Bacterial viability was also assessed with the resazurin reduction assay (n = 3).