Figure 1.
The Characterization of Human iNPCs Converted from a Small Volume of Peripheral Blood
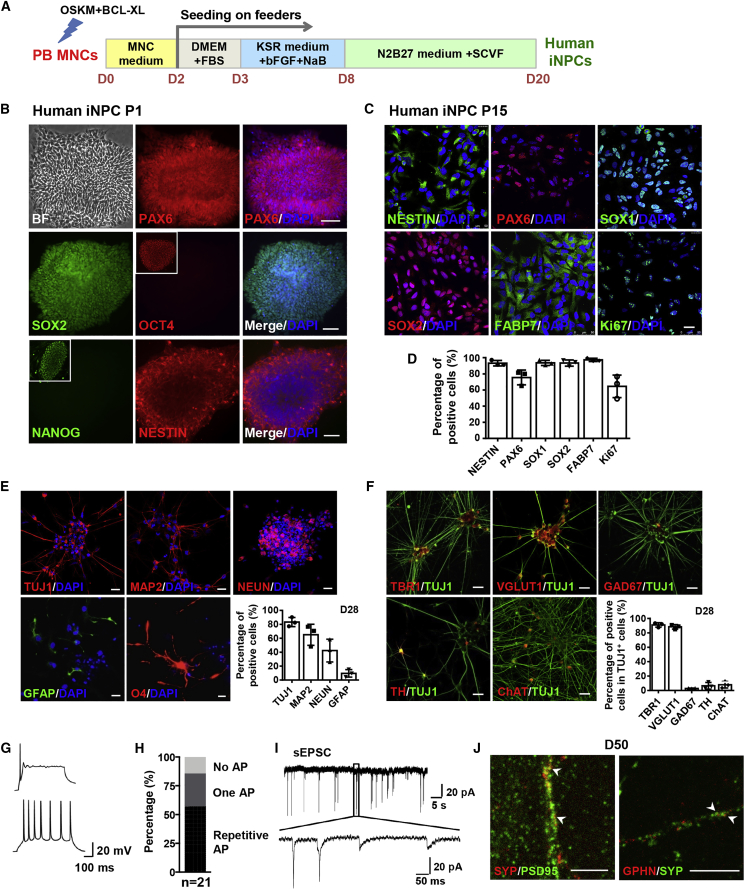
(A) Schematic representation of the approach used to direct the conversion of PB MNCs into iNPCs.
(B) Immunofluorescence analysis of human iNPCs at passage 1. Note the representative OCT4+ and NANOG+ iPSC colonies in outlined regions as positive controls.
(C) Immunofluorescence analysis of human iNPCs at passage 15.
(D) Quantification of the results shown in (C).
(E) Immunofluorescence analysis of human iNPC-derived neurons and astrocytes as at day 28, and oligodendrocytes at day 35, respectively, and corresponding differentiation efficiency.
(F) Immunofluorescence analysis of the subtypes of human iNPC-derived neurons and corresponding differentiation efficiency at day 28.
(G) Representative traces of single AP (top) and repetitive AP firing (bottom) of human iNPC-derived neurons at day 50 in response to step current injection.
(H) Percentages of human iNPC-derived cells with no AP, single AP or repetitive firing.
(I) Representative traces of spontaneous EPSCs received at a holding potential of −70 mV by human iNPC-derived neurons at day 50.
(J) Immunofluorescence analysis of SYNAPTOPHYSIN (SYP) co-labeling with PSD95 or GEPHYRIN (GPHN) in human iNPC-derived neurons at day 50. Arrowheads indicate the co-localization of pre- and postsynaptic dots.
Cell nuclei were counterstained with DAPI. Scale bars, 100 μm (B), 25 μm (C, E, F), 5 μm (J). n = 3 independent experiments. Data are represented as scatterplots with mean ± SD. Related to Figures S1 and S2.