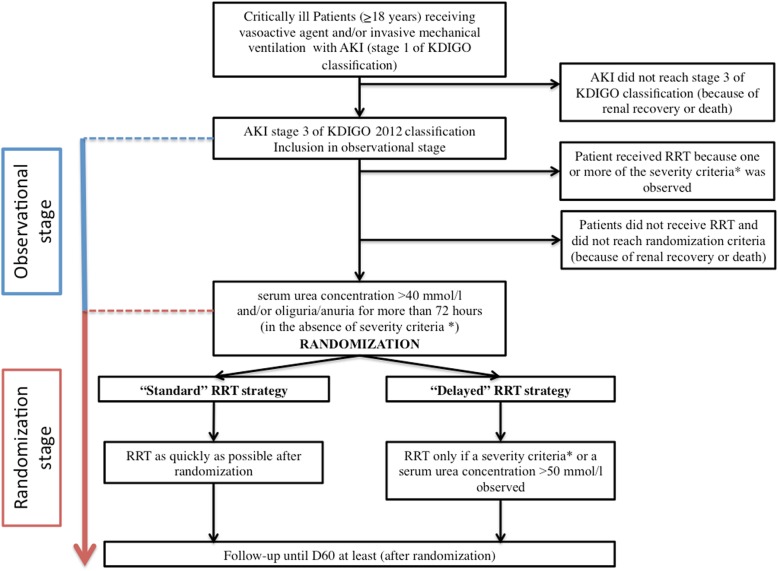
Fig. 1.
Study design. The AKIKI 2 trial is composed by 2 stages (observational and randomization stages). *Severity criteria which make considering renal replacement therapy (RRT) initiation (see Table 1): serum potassium concentration > 6 mmol/l, serum potassium concentration > 5.5 mmol/l persisting despite medical treatment, arterial blood pH < 7.15 in a context of pure metabolic acidosis (PaCO2 < 35 mmHg) or in a context of mixed acidosis with a PaCO2 > 50 mmHg without the possibility of increasing alveolar ventilation, acute pulmonary edema due to fluid overload despite diuretic therapy leading to severe hypoxemia requiring oxygen flow rate > 5 l/min to maintain SpO2 > 95% or FiO2 > 50% under invasive or non-invasive mechanical ventilation