Figure 3.
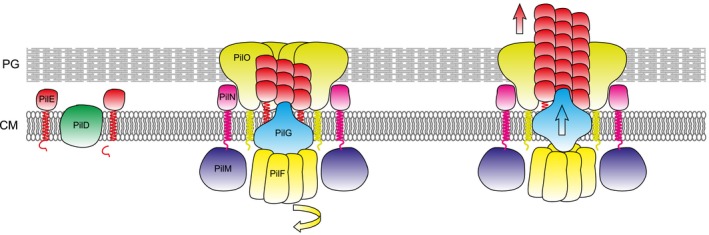
Molecular mechanisms of Tfpa assembly in monoderms: a working model. The model involves three steps, from left to right. The S. sanguinis nomenclature has been used here for the sake of clarity, and the same colour code than in Fig. 2 has been used. (1) Prepilins, which are translocated across the CM via the SRP‐Sec pathway, are processed by the prepilin peptidase PilD and constitute a reservoir of mature subunits, ready to be polymerised into filaments. (2) Filament assembly involves extrusion of the pilins from the CM and their polymerisation at the base of a growing filament, which is performed by a sub‐complex consisting of PilF‐PilG‐PilM‐PilN‐PilO. The topology of this sub‐complex is based on existing literature. ATP binding, hydrolysis and release drive a clockwise rotation of central sub‐pores in the PilF ATPase (indicated by the yellow arrow). (3) This rotatory movement thrusts the interacting PilG platform protein upwards (cyan arrow) and rotates it clockwise, before it falls back into the CM. This rotational motion pushes pilins out of the CM (red arrow) and allow their polymerisation in a right‐handed helical filament. Although the PilT retraction motor is not represented on this figure, it is thought that ATP binding, hydrolysis and release drive a counterclockwise rotation of PilT (hence reverse to PilF), which wrenches PilG downwards and facilitates PilE depolymerisation from the filament. CM, cytoplasmic membrane; PG, peptidoglycan.
