Table 1.
Optimisation of the photocatalytic isomerisation of E
‐1→Z
‐1.[a]
|
Entry |
Catalyst |
Solvent |
t [h] |
Irradiation wavelength [nm] |
Z/E ratio[b] |
|---|---|---|---|---|---|
|
1 |
– |
cyclohexane |
24 |
365 |
12:88 |
|
2 |
(−)‐riboflavin |
MeCN |
24 |
450 |
0:100 |
|
3 |
Ir(ppy)3 |
MeCN |
24 |
450 |
48:52 |
|
4 |
benzil |
MeCN |
24 |
402 |
1:99 |
|
5 |
thioxanthone |
cyclohexane |
24 |
402 |
89:11[c] |
|
6 |
benzophenone |
MeCN |
24 |
365 |
93:7[c] |
|
7 |
benzophenone |
cyclohexane |
24 |
365 |
95:5 |
|
8 |
benzophenone |
cyclohexane |
12 |
365 |
95:5 |
|
9 |
benzophenone |
cyclohexane |
6 |
365 |
95:5 |
|
10 |
benzophenone |
cyclohexane |
2 |
365 |
95:5 |
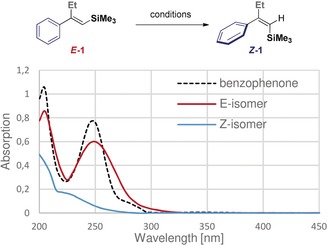
[a] Reactions were performed in degassed solvent on a 0.1 mmol scale at ambient temperature under argon atmosphere, using 5 mol % of the catalyst. [b] Z/E selectivity determined by 1H NMR spectroscopy. [c] 1H NMR spectrum of the crude reaction mixture confirmed partial decomposition.
