Figure 1.
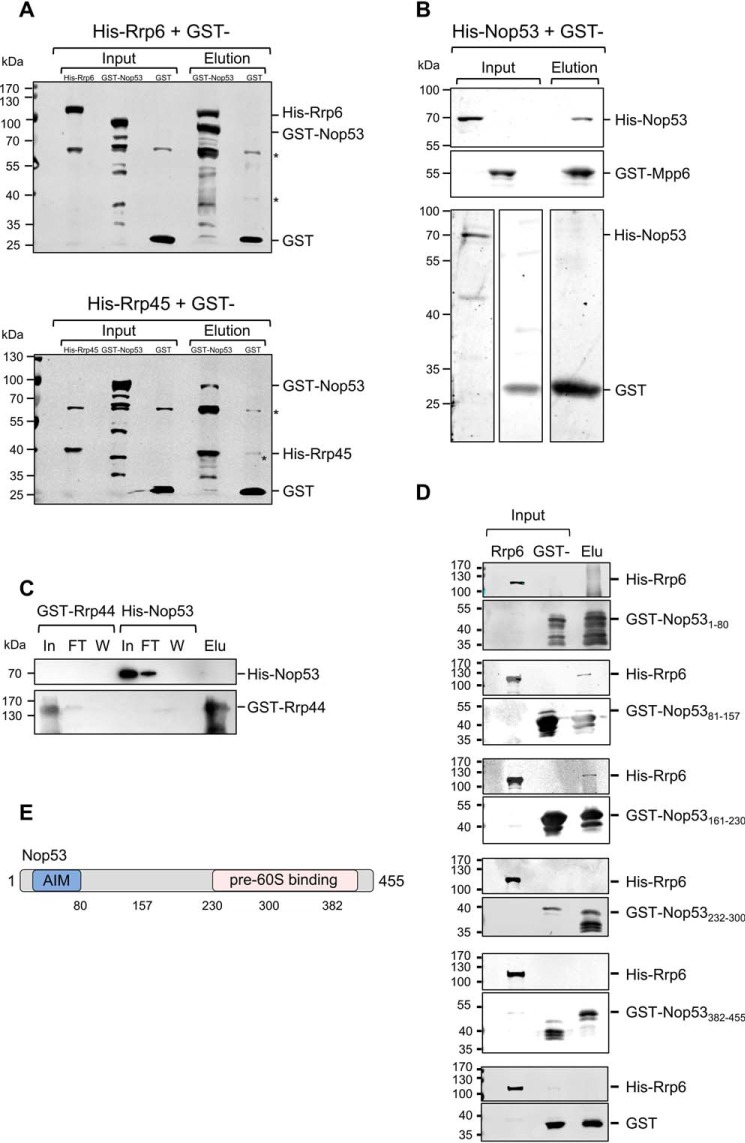
Nop53 interacts with the exosome and with exosome cofactors. GST pulldown assays were performed to test the interaction between Nop53 and the exosome components Rrp44 and Rrp45 and with the exosome cofactor Mpp6. A, GST–Nop53 immobilized in GSH-Sepharose beads pulled down His–Rrp6 and His–Rrp45. GST was used as negative control of interaction. *, E. coli proteins nonspecifically recognized by anti-GST antibody. B, GST–Mpp6, but not GST, also pulled down His–Nop53, whereas GST–Rrp44 did not (C). D, Rrp6 interacts with the N-terminal half of Nop53. The interaction between different GST-fused Nop53 truncation mutants was tested against His–Rrp6 by GST pulldown assay indicating that only the N-terminal region of Nop53 is able to interact with the exosome catalytic subunit Rrp6. GST was used as a negative control. Total extract of cells expressing His–Rrp6 was incubated either with purified GST or GST–Nop53 mutants. In, input; FT, flow-through; W, wash; Elu, elution. E, schematic representation of Nop53 with the relative positions of the amino acids indicated in the truncation mutants.