Figure 5.
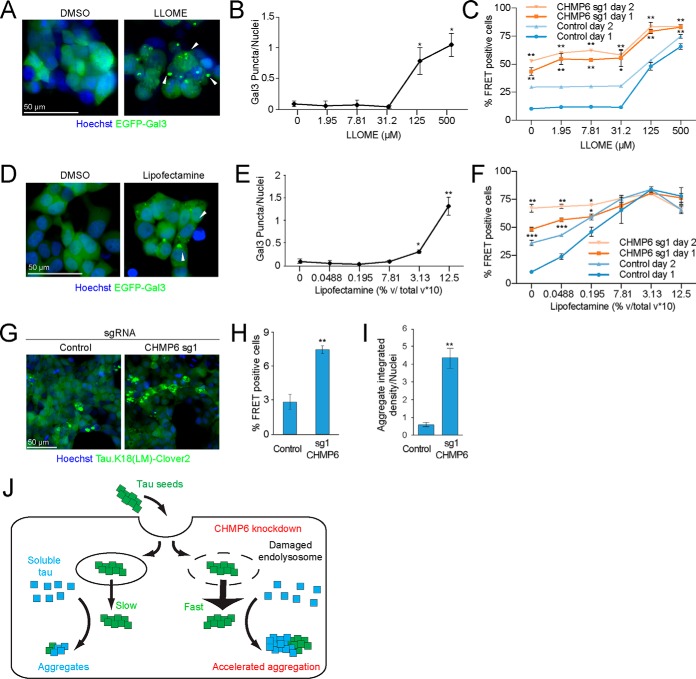
Small molecules damage endolysosomal compartments and phenocopy the acceleration of the prion-like propagation of tau aggregation following CHMP6 knockdown. A, treatment with the lysosomotropic drug LLOME damages endolysosomal vesicles. Representative fluorescence microscopy images of CRISPRi-HEK293T cells expressing the EGFP-Gal3 reporter treated with DMSO (left) or 500 μm LLOME (right) for 24 h. B, quantification of EGFP-Gal3 puncta divided by number of nuclei in fluorescence microscopy images shown in A. Error bars represent mean ± S.D. for n = 3 technical replicates (with at least 50 nuclei per image). ***, p < 0.001 (two-tailed Student's t test for comparison to DMSO control). C, LLOME treatment accelerates the prion-like propagation of tau aggregation. % FRET-positive cells transduced with control (blue) or CHMP6 sgRNA (orange) were analyzed 24 (dark line) or 48 (light line) h following co-treatment with DMSO or increasing concentrations of LLOME and tau fibrils. Error bars represent mean ± S.D. for n = 3 technical replicates. *, p < 0.05; **, p < 0.01 (two-tailed Student's t test for comparison to the values for nontargeting control sgRNA of the same day). D, Lipofectamine treatment damages endolysosomal vesicles. Representative fluorescence microscopy images of CRISPRi-HEK293T cells expressing the EGFP-Gal3 reporter treated with DMSO (left) or 1.25% (v/v) Lipofectamine 2000 (right) for 6 h. E, quantification of EGFP-Gal3 puncta divided by number of nuclei in fluorescence microscopy images shown in D. Error bars represent mean ± S.D. for n = 3 technical replicates. ***, p < 0.001 (two-tailed Student's t test for comparison to no Lipofectamine treatment). F, Lipofectamine treatment accelerates the prion-like propagation of tau aggregation. % FRET-positive cells transduced with control (blue) or CHMP6 sgRNA (orange) were analyzed 24 (dark line) or 48 (light line) h following co-treatment with PBS or increasing concentrations of Lipofectamine 2000 and tau fibrils. Error bars represent mean ± S.D. where n = 3 technical replicates. *, p < 0.05; **, p < 0.01; ***, p < 0.001 (two-tailed Student's t test for comparison to the values for nontargeting control sgRNA of the same day). G–I, CHMP6 knockdown increases the prion-like propagation tau aggregation when seeding with Alzheimer's patient brain extracts. G, representative images of FRET reporter cells transduced with control (left) or CHMP6 sgRNA (right) and treated with Alzheimer's patient brain extract after 5 days. H, quantification of images in G. Tau aggregates were quantified by integrated density across the entire image and divided by the total nuclei per image. Error bars represent mean ± S.D. for n = 3 images per condition (with at least 50 nuclei per image). **, p < 0.01 (two-tailed Student's t test for comparison to nontargeting control sgRNA) (I) % FRET-positive cells 5 days following treatment with Alzheimer's patient brain extract. Error bars represent mean ± S.D., where n = 3 technical replicates. **, p < 0.01 (two-tailed Student's t test for comparison to nontargeting control sgRNA). J, model for the increased tau aggregation via CHMP6 knockdown and generation of damaged endolysosomes.