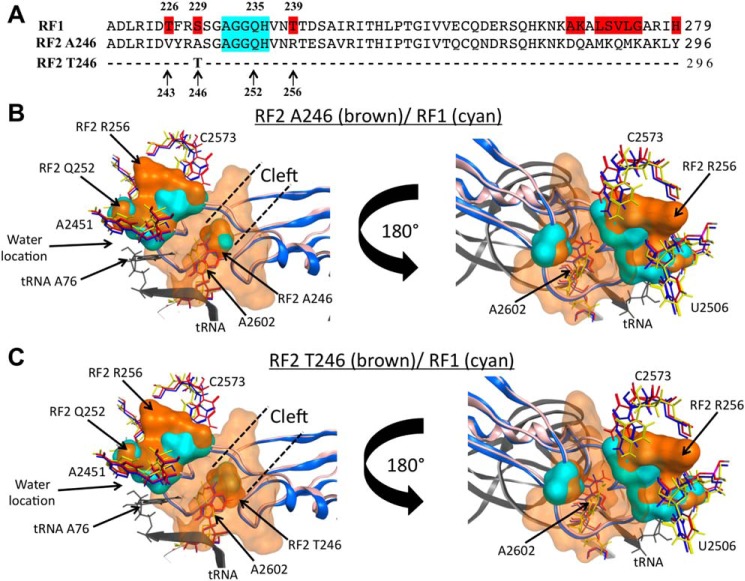
Figure 5.
Structural comparisons between RF2 and RF1 protein molecules. A, comparison of the primary sequences of the sections 220–279 and 237–296 of RF1 and two RF2 variants, respectively, which correspond to the tips of domain 3 that hold the GGQ functional motif (highlighted in cyan). Residues highlighted in red on the RF1 sequence are different between the three proteins. B and C, molecular modeling was performed as indicated under “Experimental procedures.” The structures show a view of overlapping molecular densities of the tips of domain 3 of (B) RF2 Ala-246/Arg-256 protein variant, and (C) Thr-246/Arg-256 (both in brown color), with RF1 (cyan color), all accommodated at the PTC A-site. The structural conformations of the A2602 nucleotide (sticks) observed in the presence of RF2 (red, PDB ID 5CZP) (7), RF1 (blue, PDB 5J30) (7), or with the TnaC peptide (yellow, PDB 6I0Y) (25) are also shown. The structural conformations of C2573 and U2506 acquired with RF2 (red), RF1 (blue), or TnaC (green) are shown as well. For both (B) and (C) panels, a tRNA located at the PTC P-site is shown in black. CCG MOE and Chimera were used to obtain the figures.