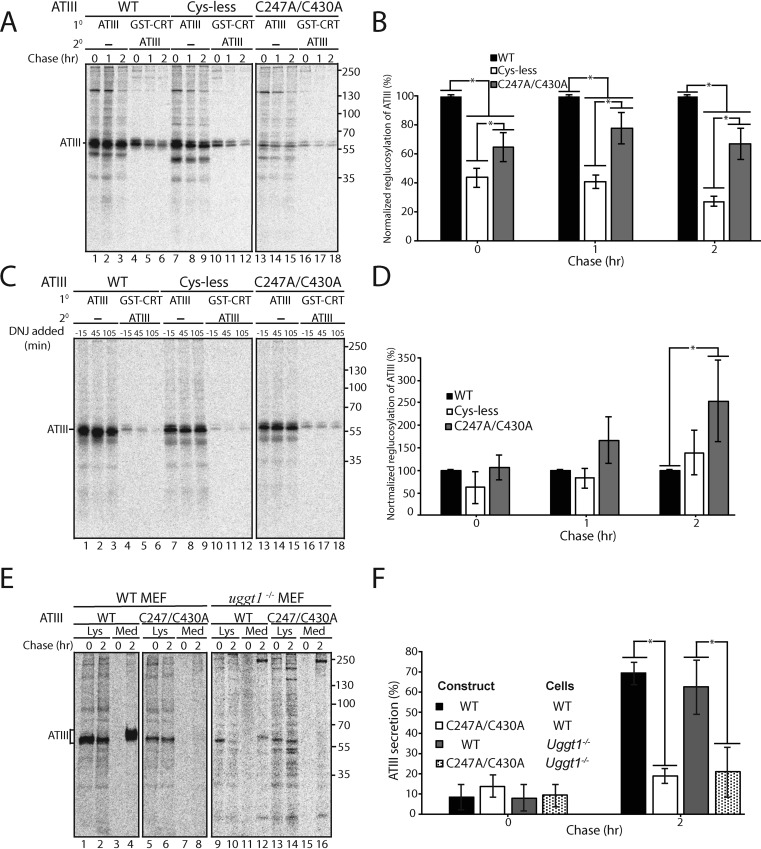
Figure 5.
Retention of C247A/C430A in the ER does not require UGGT1. A, ATIII variants were expressed in MI8-5 CHO cells. 30 min prior to the pulse and throughout the chase, cells were treated with 0.5 mm DNJ. Cells were radiolabeled with [35S]Cys/Met for 30 min and chased for the indicated times. At each time point, cells were lysed in MNT buffer. 80% of the cell lysate was affinity-purified with GSH S-transferase–tagged calreticulin (GST-CRT), whereas 20% of the cell lysate was immunopurified with anti-Myc antibody. GST-tagged calreticulin affinity purifications were then eluted in buffer containing 1% SDS, diluted in MNT, and immunopurified using anti-Myc tag antibody. Samples were resolved by reducing 9% SDS-PAGE. B, quantification of reglucosylation in A. Percentage reglucosylation for each ATIII variant was calculated by quantifying the bands corresponding to ATIII, multiplying the lysate band by 4, and dividing the amount of ATIII in the sequential IP by the amount of ATIII in the nonsequential IP at each time point. All reglucosylation values are normalized to WT ATIII. C, same as A, except DNJ was added 15 min prior to each time point, not throughout the experiment. D, quantification of reglucosylation in C. Percentage reglucosylation was calculated as described in B. E, ATIII and ATIII C247A/C430A were expressed in both WT and UGGT1−/− MEF cells. Cells were radiolabeled with [35S]Cys/Met for 30 min and chased for the indicated times. At each time point, cell lysate and media were collected and processed as described in the legend to Fig. 1A. F, quantification of ATIII secretion from E. The lysate and media were quantified, and ATIII secretion is presented as a percentage of ATIII in the media to ATIII in the 0-h lysate. All experiments are representative of three independent experiments. Error bars, S.D.