Figure 8.
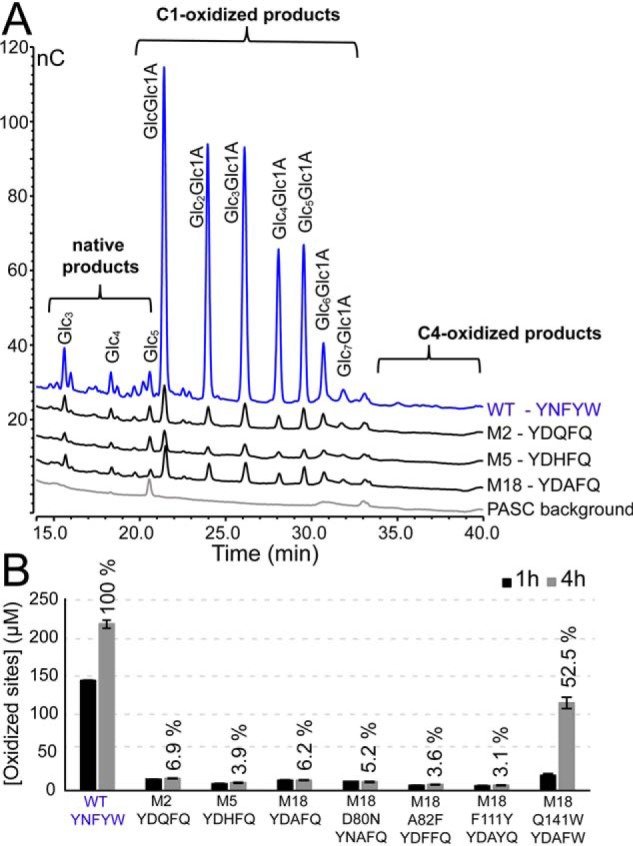
Residual cellulolytic activity. ScLPMO10C WT and mutants were incubated at 1 μm with 5 g/liter PASC and 1 mm ascorbic acid at 40 °C and in 50 mm sodium phosphate (pH 6.0). A shows oxidized products after ∼24 h of incubation, varying from the dimer (GlcGlc1A) to the octamer (Glc7Glc1A), generated by ScLPMO10C WT, M2, M5, and M18. The background signal of the substrate (PASC) incubated with LPMO in the absence of reductant is also shown. Note that the size of the peaks of the WT chromatogram was reduced by 25% before making the plot. Regions where C4-oxidized products would elute are marked. B shows the amount of soluble oxidized sites in aliquots that were withdrawn after 1 and 4 h of incubation; in this case oxidized products were quantified after degrading them to a mixture of oxidized dimers (GlcGlc1A) and trimers (Glc2Glc1A) only, by treatment with the endoglucanase TfCel5A. Relative activities were calculated relative to the amount of product generated by the WT enzyme after 4 h (100%). Testing of the cellulolytic activity of M18 with Avicel gave similar results. All reactions were performed in triplicate, and the error bars show ± S.D. (n = 3).
