Figure 2.
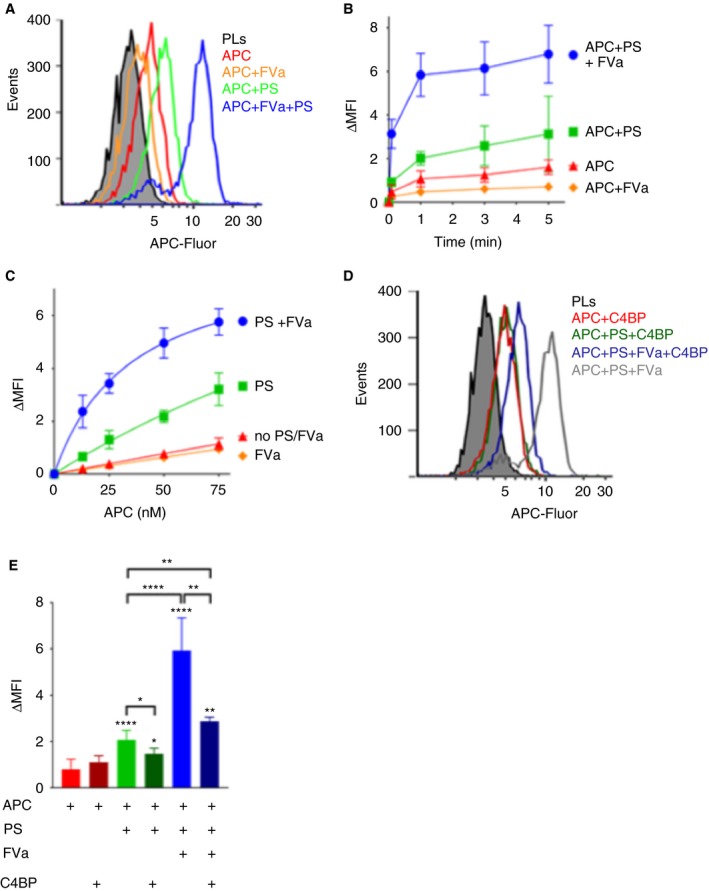
FVa in synergy with protein S enhances the binding of APC to negatively charged phospholipids. A, Representative histograms of binding of 50 nmol/L APC‐Fluor to phospholipid‐coated magnetic beads in the presence and absence of 100 nmol/L protein S and/or 25 nmol/L FVa using flow cytometry. B, The APC association to phospholipid coated magnetic beads was characterized over time. The MFI of the phospholipid‐coated beads was measured at 0, 1, 3, and 5 min after addition of APC. C, APC binding was analyzed at increasing concentrations (0‐75 nmol/L) in the presence and absence of 100 nmol/L protein S and/or 25 nmol/L FVa. Half maximal binding of APC‐Fluor was estimated to be 34.7 nmol/L in the presence of protein S and FVa. D, Representative histograms showing the effects of C4BP upon APC binding to phospholipids. Binding of 50 nmol/L APC‐Fluor to phospholipid‐coated magnetic beads was measured in the presence and absence of 100 nmol/L protein S preincubated with 200 nmol/L β‐chain containing C4BP and/or 25 nmol/L of coagulation factor FVa using flow cytometry. E, Quantification plot of binding of 50 nmol/L APC‐Fluor to phospholipid‐coated magnetic beads in the presence or absence of 100 nmol/L free or C4BP‐bound protein S and/or 25 nmol/L FVa. MFI was measured 1 min after addition of APC. ΔMFI corresponds to the MFI obtained after subtracting the auto fluorescence from the phospholipid coated beads. Data are presented as mean ± SD; n ≥ 3. *P < .05, **P < .01, ****P < .0001 according to Mann‐Whitney tests compared to APC alone, unless otherwise stated
