Scheme 3.
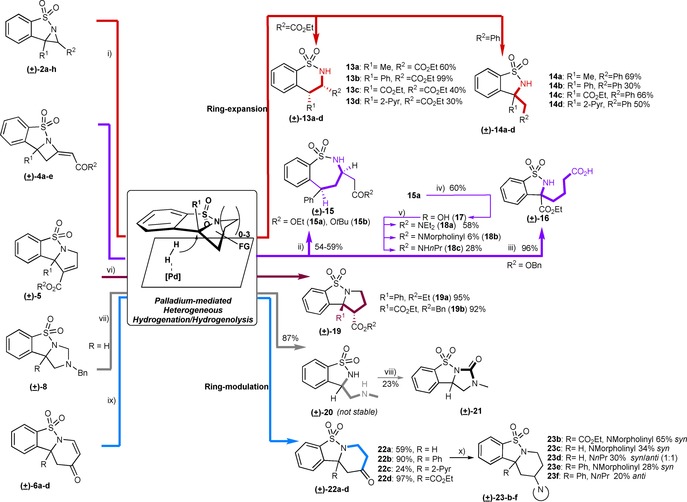
Ring‐expansion and modulation reactions through palladium‐mediated heterogeneous hydrogenation/hydrogenolysis. Conditions: i) 10 mol % Pd/C, EtOH/EtOAc,1 atm H2; ii) 14 mol % Pd/C, EtOH/EtOAc, 7.5 atm H2; iii) Pearlman′s catalyst (20 wt %., 9 mol %), MeOH/EtOAc, 2 h; iv) aq. NaOH/THF/MeCN, 19 h; v) first, (COCl)2 or SOCl2 in CHCl3, 60 °C; then, corresponding amine in THF at 0 °C; vi) Pd(OH)2/C, H2; vii) 10 mol % Pd/C, 7 atm H2, 16 h; viii) Et3N, CDI, MeCN, 16 h; ix) 10 mol % Pd/C H2, EtOH (+EtOAc for 22 d). For 22a: additional step of DMP/NaHCO3, DCM 0° C, 3 h; x) NaB(OAc)3H, corresponding amine, 4 Å MS, 1,2‐DCE, 16–48h.
