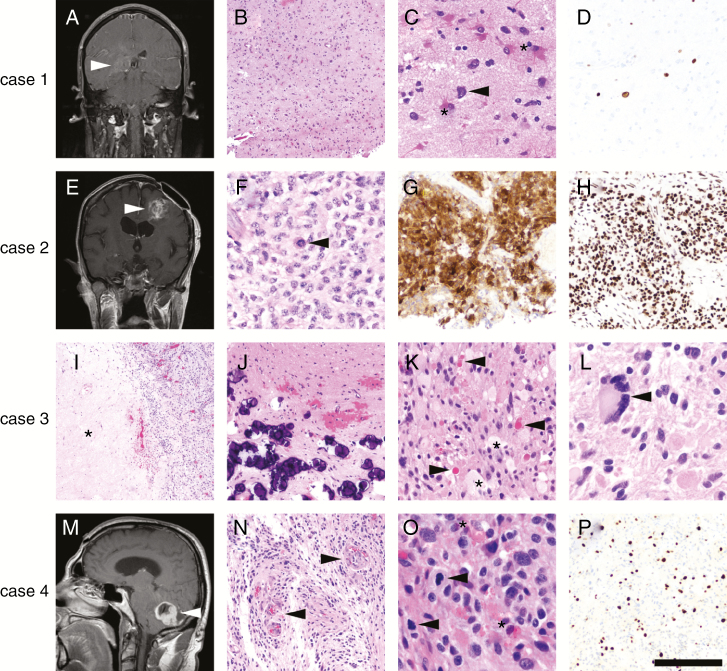
Fig. 3.
Examples of the impact of advanced molecular testing in brain tumors. Case 1 was a 44-year-old man with an ill-defined enhancing lesion in the right deep temporal lobe (A, arrowhead). A stereotactic biopsy contained moderately hypercellular brain tissue (B), composed mostly of reactive astrocytes (C, asterisks) and cells with highly atypical nuclei (C, arrowhead). No mitoses, necrosis, or microvascular proliferation was present in the biopsied material. Ki67 immunostain (D) showed only scattered positive cells. NGS detected mutations in TP53, PTEN, and the TERT promoter, leading to the diagnosis of GBM and sparing the patient a repeat surgery to obtain histologically diagnostic tissue. The patient was treated with RT/TMZ and survived less than a year after diagnosis. Case 2 was a 50-year-old woman in whom was histologically diagnosed a left frontal WHO grade II diffuse astrocytoma 10 years ago at an outside hospital (OSH), who now presented with recurrence and new enhancement (E, arrowhead). Histologically, the recurrent tumor was high grade with necrosis and microvascular proliferation (not shown), as well as mitoses (F, arrowhead) and nuclei that appeared more astrocytic than oligodendroglial. An immunostain for IDH1 R132H was positive (G), yet nuclear ATRX expression was retained (H). NGS confirmed the IDH1 R132H mutation and detected a TERT promoter mutation, as well as 1p/19q codeletion. The tumor was therefore reclassified as anaplastic oligodendroglioma, WHO grade III. The patient responded well to RT and PCV, with no recurrences 1 year later. Case 3 was a pathology consult from an OSH, consisting of a right parietal mass in a 25-year-old man (no radiology available). Histologically the tumor had necrosis (I, asterisk) and mitoses (not shown), but also had abundant mineralization (J), eosinophilic granular bodies (K, arrowheads), foamy lipidized cells (K, asterisks), and highly atypical cells, including multinucleated giant cells (L, arrowhead). The OSH diagnosed the tumor as a GBM, because the screening for BRAF V600E was negative. However, NGS detected a rare oncogenic variable lymphocyte receptor insertion between codons 506 and 507 of BRAF, previously described in Langerhans cell histiocytosis.88 No other alterations were detected, so the tumor was reclassified as anaplastic pleomorphic xanthoastrocytoma, WHO grade III. The patient had already been treated at the OSH with RT/TMZ based on the original GBM diagnosis, but was still alive 2 years later with no recurrences. Case 4 was a 50-year-old man with an enhancing, well-circumscribed cerebellar mass (M, arrowhead) containing microvascular proliferation (N, arrowheads), numerous mitoses (O, arrowheads), eosinophilic granular bodies (O, asterisks), and a high Ki67 proliferation index (P), but no necrosis or Rosenthal fibers. This tumor was originally diagnosed at the OSH as a GBM and treated with RT/TMZ, but NGS (performed several years later) detected an FGFR1 mutation and CDKN2A deletion, changing the diagnosis to an anaplastic pilocytic astrocytoma.12–15 The patient survived 7 years before the tumor recurred, and is still alive 2 years after re-resection (9 years total). Scale bar = 500 microns in I; 200 microns in B, G, H, J, N, P; 100 microns in C, D, K; 50 microns in F, L, O.