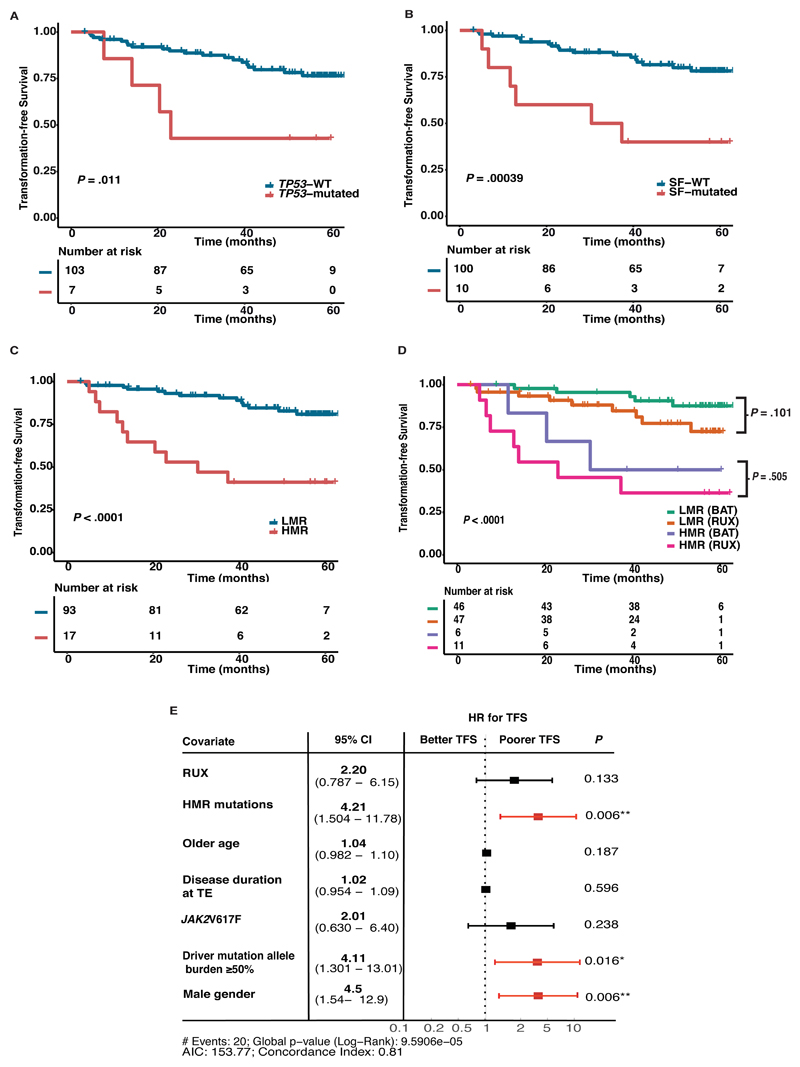
Figure 2. Kaplan-Meier curves of transformation-free survival (TFS) stratified by mutational statuses with survival estimates, reported at 4-years.
(A) TP53 mutations were associated with inferior 4-year TFS; TP53-mutated (42.9% [95% CI 9.8 – 73.4%]) versus TP53-wild type (WT) patients (79.8% [95% CI 69.7 – 86.8%]), p=0.011. (B) SF mutations conferred a poorer 4-year TFS; SF-mutated (40% [95% CI 12.3 – 67%]) versus SF-WT (81.5% [95% CI 71.4 – 88.3%]), p=0.00039. (C) Comparing patients with HMR with LMR at 4-years; HMR 41.2% (95% CI 23.3-72.7%) versus LMR 84.6% (95% CI 76.9 – 93.1%), p<0.0001. (D) Stratifying patients with high risk molecular (HMR) mutations in this study by treatment arm demonstrates no amelioration of negative impact of HMR mutation with RUX treatment; patients with HMR on RUX had TFS at 4-years of 36.4% (95% CI 26.2 – 46.6%) and on BAT 50% (29.1 – 67.7%) (p=0.505 between these arms) as compared to those without these mutations (i.e. low molecular risk, LMR) with TFS at 4-years of 84.7% (95% CI 71.6 – 92%) on RUX and of 90.6% (95% CI 78.5 – 96%) on BAT (p=0.101 between these arms). The log-rank test was used to compare survival estimates between groups. (E) Forest plot showing multivariable cox model of TFS. Covariates significant on univariate analysis were included; TP53 mutations, SF mutations, treatment arm, JAK2V617F mutation status, disease duration at trial entry (TE), age and gender. HMR mutations independently retained negative impact on TFS with a hazard ratio (HR) of 4.21, p=0.006. Treatment arm, JAK2V617F status, disease duration at TE and age were not significant but notably male gender was associated with a poorer TFS, HR 4.5, p=0.006. Driver mutation allele ≥50% was independently associated with a poorer TFS, HR 4.11, p=0.016. Age and disease duration at TE were categorized as continuous variables. CI=confidence interval; HR=hazard ratio; HMR=high molecular risk risk (SF and TP53 mutations); LMR=low molecular risk (without SF or TP53 mutations); JAK2=JAK2V617F; NDM=non-MPN (myeloproliferative neoplasm) driver mutation; SF=splicing factor mutation (SF3B1, ZRSR2, SRSF2); WT=wild type.