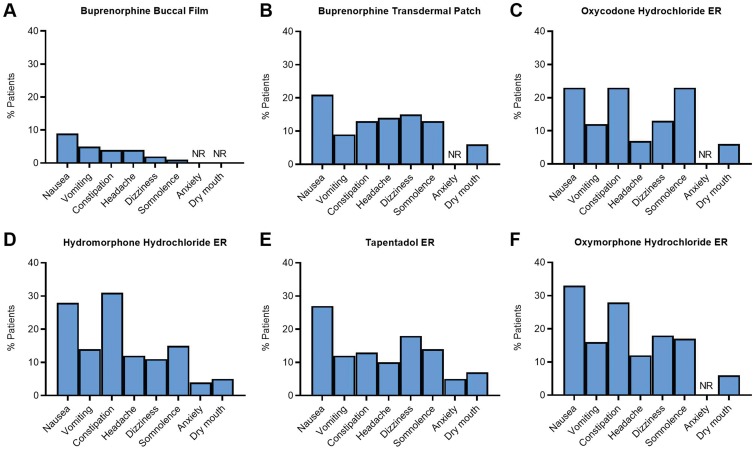
Figure 4.
Safety analysis: adverse reactions reported in clinical trials of buprenorphine formulations and common Schedule II opioids for chronic pain. The percentage of patients who reported adverse reactions in clinical trials for buprenorphine buccal film (A)28 is lower than those reported for the buprenorphine transdermal patch (B),29 oxycodone hydrochloride ER (C),79 hydromorphone hydrochloride ER (D),77 tapentadol ER (E),81 and oxymorphone hydrochloride ER (F).70
Abbreviations: ER, extended-release; NR, not reported.