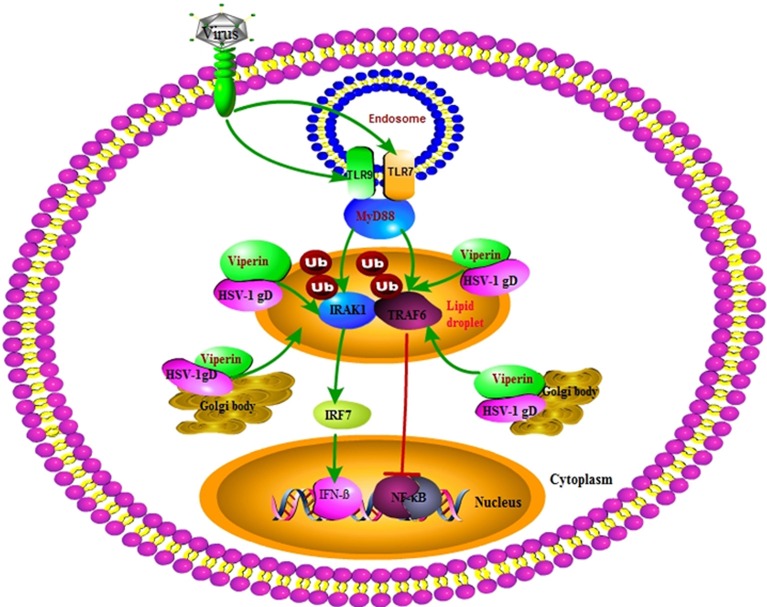
Figure 8.
Overview of the molecular mechanism of HSV-1 gD and viperin interaction. HSV-1 gD can interact with viperin, and co-localize with it at the lipid droplets and Golgi body. The gD and viperin interaction facilitates IRF7-mediated IFN-β activity by promoting viperin and IRAK1 interaction and facilitating K63-linked IRAK1 ubiquitination, whereas gD attenuates TRAF6-induced NF-κB activity by inhibiting the viperin and TRAF6 interaction, but not affecting the polyubiquitination of TRAF6. Viperin alone promotes the interaction of IRAK1 and TRAF6, which is inhibited in the presence of gD and viperin. Eventually, gD and viperin interaction is corroborated to significantly inhibit the proliferation of HSV-1.