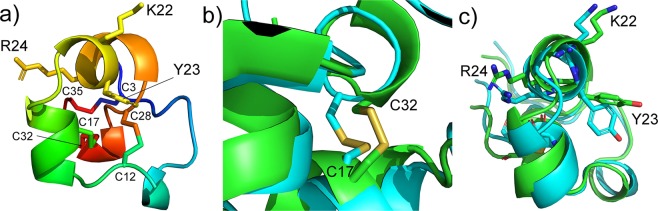
Figure 5.
Views of ShK. (a) Overall structure of ShK, colored from blue at the N-terminus to red at the C-terminus, using the PDB file 4LFQ14. The six cystines are indicated, plus three residues thought to interact with the Kv1.3 potassium channel (Lys22, Tyr23 and Arg24). (b) Closeup view of the C17-C32 disulfide. The structures shown are iterations 200 from the molecular dynamics simulations, with the ground-state (negative χ3) in green, and the alternative conformation (positive χ3) in cyan. (c) End-on view of helix 21–24, showing the positions of Lys22, Tyr23 and Arg24 in iterations 200 from the molecular dynamics simulations. The Cys17-Cys32 disulfide is just visible at the back of the helix at the bottom.