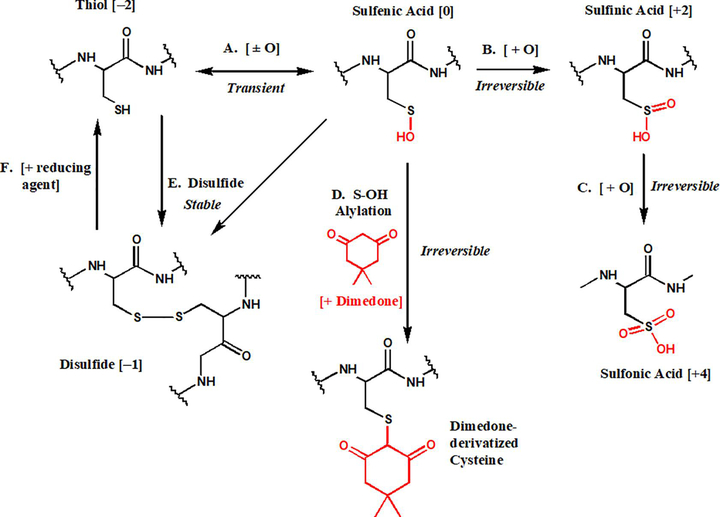
Figure 1. Redox dependent fates of cysteine residues.
Cysteine residues tonically exist in the thiol state with an oxidation state of −2. First order oxidation of cysteine residues via cysteine-S-sulfenation (A) is a transient oxidative modification leading to an oxidation state of 0. Sulfenic acids can be further oxidized to sulfinic (B) or sulfonic (C) acids, both of which are irreversible modifications, that lead to oxidation states of +2 and +4, respectively. Experimentally, a sulfenic acid can be selectively alkylated by cyclic diketones such as dimedone [11, 24, 67–68], which physically blocks subsequent redox reactions (D). A sulfenic acid can also react with a free cysteine thiol to form a disulfide bond with an oxidation state of −1 (E), yielding a stable modification that is experimentally labile to reaction with reducing agents (F).