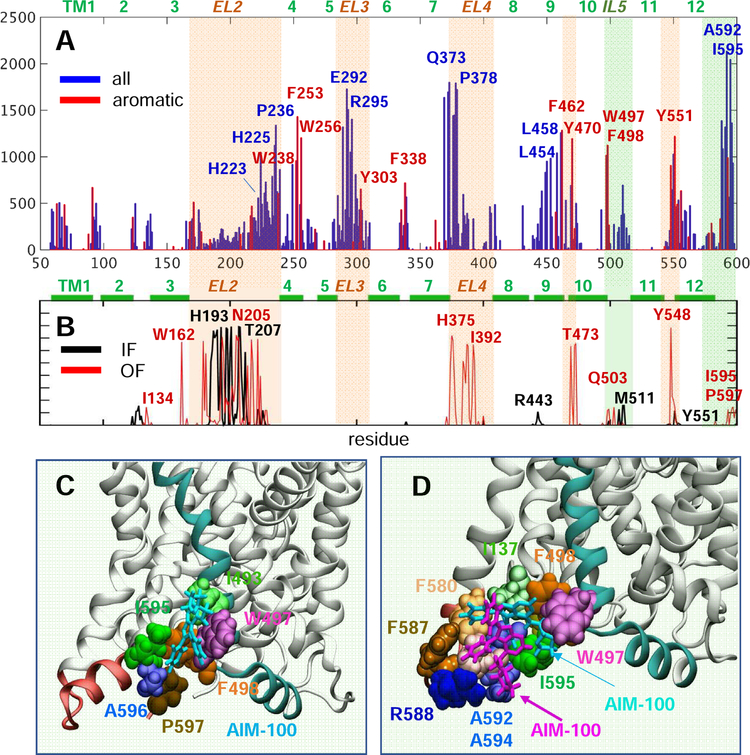
Fig. 5. Histogram of contacts between AIM-100 molecules and DAT, and a high affinity binding sites near the C-terminus.
(A) The bars display the probability distribution of residues within 3.5 Å atom-atom distance from AIM-100, observed in the MD simulations of all four DAT trimers (over 2,000 frames). We note the preponderance of aromatic residues (highlighted in red). (B) Results from the MD simulations of IF (black) and OF (red) monomers in the presence of AIM-100s. Light orange and green shades in A and B highlight EC and IC-exposed regions distinguished by high-binding affinity. (C-D) The region between the C-terminus (pink) and TM10-IL5 (cyan) harbors a favorable AIM-100 binding site for (C) one AIM-100 (cyan sticks) or (D) two AIM-100s (cyan and purple sticks). This type of binding was consistently observed in all three OF trimer simulations, with I595 interacting intermittently with W497 or F498. Panels C and D display snapshots from the simulations of trimer-Y303 and -W238, both in OF state.