Abstract
Background:
Patients may experience improved quality of life (QoL) without complete clearance of skin disease. The Cutaneous Dermatomyositis Disease Area and Severity Index-Activity (CDASI-A) score correlates with the Symptoms and Emotions subscales of Skindex-29, a measure of QoL, down to CDASI-A scores of 7 (for Symptoms) and 10 (for Emotions).
Objectives:
Our goal is to define an important change in disease activity, as measured by CDASI-A, that results in meaningful change in QoL in DM patients.
Methods:
In 103 patients, we assessed the percent change and actual change in CDASI-A needed to achieve meaningful improvement in QoL using linear regression models.
Results:
We found meaningful improvement is 7.86 (P<.0001) points in Symptoms and 10.29 (P<.0001) points in Emotions, after correlating Skindex-29 to an established definition of meaningful change in the Dermatology Life Quality Index (DLQI). For patients with initial CDASI-A scores >14, a 40% change in CDASI-A between the first two visits suggests a meaningful change in Skindex-29. In patients with moderate initial CDASI-A (15-26), the change in CDASI-A resulting in a meaningful change in Symptoms and Emotions was 6 (P=.0002) and 7 (P=.0007), respectively. For initial CDASI-A scores in the severe range of (27-35), an improvement in CDASI-A by 11 (P=.0301) and 9 (P=.0212) points leads to meaningful change in Symptoms and Emotions, respectively.
Conclusions:
In patients with an initial CDASI-A of >14, a 40% change in CDASI-A can be used to indicate meaningful change in QoL in future DM trials.
Introduction
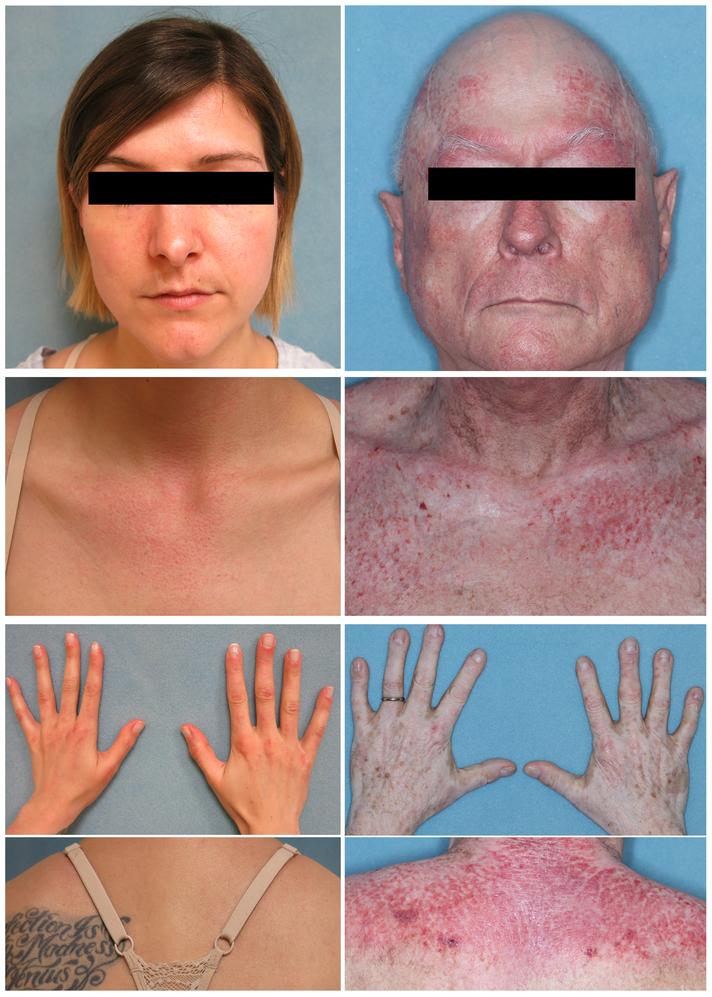
Dermatomyositis (DM) is a heterogenous autoimmune disease characterized by varying degrees of skin, joint, and muscle inflammation (Figure 1).1,2 The majority (94%) of DM patients have cutaneous manifestations, which can significantly impair quality of life (QoL).1,3 However, patients may experience a positive change in QoL despite a comparatively smaller improvement in skin disease. The Skindex-29 and Dermatology Life Quality Index (DLQI) are standardized and validated measures of QoL for clinical trials and correlate with Cutaneous Dermatomyositis Disease Area and Severity Index-Activity (CDASI-A) scores in patients with DM. The lowest CDASI-A score at which the CDASI-A correlates well with QoL is 4 for the DLQI, and 10, 8, and 7 for the Skindex-29 subscales of Emotions, Functioning, and Symptoms respectively, with little further improvement in QoL below these scores.4
Figure 1:
Two patients with dermatomyositis. On left column: A 33-year-old female with a CDASI-A of 20 which indicates moderate disease. On the right column: A 73-year-old male with severe skin disease and a CDASI-A of 44.
Minimal clinically important difference (MCID) is synonymous to a meaningful change in Qol which reflects an improvement in symptoms or function from the patient’s perspective.5,6 The meaningful change for the DLQI is a 5-point difference for inflammatory skin diseases.6 We aimed to define this meaningful change in Skindex-29 scores. In addition, we aimed to identify the change in CDASI-A scores between two consecutive visits that results in a meaningful improvement in QoL.
Materials and Methods
Adult patients (greater than 18 years of age) seen in the outpatient Autoimmune Disease Clinic at the University of Pennsylvania from 2008 to 2018 were screened and consented for enrollment into a dermatomyositis (DM) database. These patients had clinical or histological evidence of DM and were asked to answer quality of life surveys in the form of the Skindex-29 and DLQI (Dermatology Life Quality Index). Patients were evaluated by a board-certified medical dermatologist (VPW) using the CDASI at each study visit. All participants were consented according to an IRB-approved protocol. Data was entered into REDcap, a secure web-based database. We used Graphpad Prism for our calculations, using a significance level of p≤0.05.
Only patients who answered the Skindex-29 and DLQI to completion in both the first and second visits were included. Out of 252 subjects, 97 were excluded because they did not return for a second visit. Out of 155 patients, 105 patients filled out both the Skindex-29 and DLQI to completion. 40 patients out of 252 subjects in our database were excluded due to failure to complete the DLQI in either the first or second visit. Those who failed to fill out the Skindex-29 or DLQI questionnaires to completion in both visits (50 out of 155 patients) were excluded, along with those with an initial CDASI score of 0 (n=2), resulting in the inclusion of 103 patients.
CDASI
The CDASI is a validated disease assessment tool used to capture the extent of cutaneous disease activity and damage, and monitor disease over time with regards to activity in the form of erythema, scale, erosion or ulceration, and damage with regards to poikiloderma and calcinosis in patients with dermatomyositis. The tool specifies 15 anatomical locations on the body for the physician to evaluate. The CDASI gives two scores to each patient; one score for activity (erythema, scale, erosion, ulceration) and one score for damage (poikiloderma or calcinosis). The tool additionally measures Gottron’s papules, periungual changes and alopecia as part of activity of DM. Activity scores can range from 0 to 100, while damage can range from 0 to 32, with higher scores indicating worse disease severity. Our study examined the activity component of the CDASI. Patients with an initial CDASI-A score of 0 were excluded.
DLQI
The DLQI is a concise, validated instrument developed in 1994 that is used to assess the quality of life in patients with dermatologic conditions.7 Its 10 questions span subjects pertaining to how one’s skin condition affects personal relationships, work, daily activities, and perceptions of skin symptoms. The DLQI has a greater focus on how disease affects a person’s ability to function in daily activities than on one’s emotions. An increase in score indicates worsened quality of life. After a preliminary abstract demonstrated that a change of 5 in the DLQI leads to a meaningful change, studies and therapeutic guidelines involving inflammatory skin conditions have used this value.8,6
Skindex-29
The Skindex-29 is a validated measure of QoL based on cutaneous disease. Scoring is categorized into 3 subscales: Functioning, Emotions, and Symptoms, with each question having 5 choices correlating to a score of 0, 25, 50, 75, or 100.9 An increase in score also indicates worsening quality of life.
Derivation of Meaningful Change in the Skindex-29
Minimal clinically important difference is an important or meaningful change from the patient’s perspective.5,6 By this definition, meaningful change is equivalent to MCID. The difference in DLQI scores were correlated to the difference in Skindex-29 subscales in a linear regression to identify what difference in quality of life score between two consecutive visits constitutes a meaningful change for those patients with moderate to severe disease. A change of 5 in the DLQI scores was used to derive this value in the Skindex-29. Study participants with at least two documented visits were included.
Defining Important Change in CDASI Activity Score
Percent changes in CDASI-A from Visit 1 to Visit 2 were calculated, with a negative percentage indicating an improvement in skin activity. The difference in Skindex-29 subscales scores between visit 1 and 2 were calculated and compared to percent changes and absolute score changes in CDASI-A. The Functioning subscale was not analyzed as it poorly correlates with CDASI scores.3 Linear regression models were created for the difference in Skindex-29 subscales of Symptoms and Emotions between visit 1 and 2. Patients were stratified into mild and moderate to severe disease by initial CDASI-A scores. CDASI-A scores of ≤14 were considered mild disease, while scores >14 denoted moderate to severe disease activity.10 CDASI-A scores >26 were also analyzed in our data because of prior research by Chock et al11 stating that a CDASI-A score of 25.6 ± 8.9 could be interpreted as moderate disease. We also examined changes in CDASI-A scores in categories with higher cut-off values to evaluate patients with more severe disease (Tables 2, 3). Figure 1 shows two patients with moderate and severe disease and their correlating CDASI-A scores.
Table 2:
Percent change in CDASI-A scores associated with meaningful improvement in Skindex-29 subscales (Emotions and Symptoms) in DM patients entering with a range of CDASI activity scores
| Initial CDASI Score >14 (n=64) |
Percent Change | Slope (95% CI) | p-value |
|---|---|---|---|
| Skindex-29 Symptoms | 31.67 | .25 (.14-.35) | <.0001 |
| Skindex-29 Emotions | 34.96 | .29 (.17-.42) | <.0001 |
| Initial CDASI Score (15-26) (n=34) |
|||
| Skindex-29 Symptoms | 27.25 | .29 (.14-.44) | .0004 |
| Skindex-29 Emotions | 35.45 | .29 (.13-.45) | .0009 |
| Initial CDASI Score >26 (n=30) |
|||
| Skindex-29 Symptoms | 49.34 | .16 (−.005-.32) | .0568 |
| Skindex-29 Emotions | 34.23 | .30 (.08-.52) | .0102 |
| Initial CDASI Score (27-35) (n=18) |
|||
| Skindex-29 Symptoms | 36.68 | .21 (.02-.41) | .0342 |
| Skindex-29 Emotions | 23.10 | .34 (.05-.53) | .0261 |
Table 3:
Absolute change in CDASI-A scores associated with meaningful improvement in Skindex-29 subscales (Emotions and Symptoms) in DM patients entering with a range of CDASI scores
| Initial CDASI Score >14 (n=64) |
Difference | Slope (95% CI) | p-value |
|---|---|---|---|
| Skindex-29 Symptoms | 10.27 | .77 (.35-1.18) | .0005 |
| Skindex-29 Emotions | 10.38 | .99 (.52-1.47) | <.0001 |
| Initial CDASI Score (15-26) (n=34) |
|||
| Skindex-29 Symptoms | 5.61 | 1.40 (.71-2.10) | .0002 |
| Skindex-29 Emotions | 7.37 | 1.40 (.63-2.16) | .0007 |
| Initial CDASI Score >26 (n=30) |
|||
| Skindex-29 Symptoms | 19.37 | .41 (−.06-.87) | .0834 |
| Skindex-29 Emotions | 13.26 | .78 (.14-1.41) | .0183 |
| Initial CDASI Score (27-35) (n=18) |
|||
| Skindex-29 Symptoms | 10.76 | .73 (.08-1.38) | .0301 |
| Skindex-29 Emotions | 8.79 | 1.17 (.20-2.14) | .0212 |
Results:
103 patients were included in this retrospective study of prospectively collected data after excluding patients due to no initial disease activity or failure to complete either the Skindex-29 or DLQI. The median time between the first two visits was 126 days (IQR= 17–294 days). We found that meaningful improvement in the Symptoms and Emotions subscales is 7.86 (P<0.0001) and 10.29 (P<0.0001) points, respectively when comparing the Skindex-29 to the Dermatology Life Quality Index (DLQI) (Table 1). In patients with an initial CDASI-A score of >14, approximately a 40% change in CDASI-A score between the first two visits correlated with a meaningful change in QoL. Specifically, a 32% change in CDASI-A score leads to an improvement in the Symptoms subscale of the Skindex-29, while a 35% change in CDASI-A score results in a meaningful change in the Emotions subscale (Table 2). A greater percent change in score between the first two visits is needed to result in a meaningful change in QoL when including patients with lower baseline CDASI-A scores.
Table 1:
Meaningful Change in Skindex-29 Relative to Meaningful Change in the Dermatology Life Quality Index
| All CDASI Scores (n=103) |
Change in QoL** | Slope (95% CI) | p-value |
|---|---|---|---|
| Skindex-29 Symptoms | 7.32 | 1.46 (.95-1.98) | <.0001 |
| Skindex-29 Emotions | 9.78 | 1.95 (1.37-2.53) | <.0001 |
| Initial CDASI Score >14 (n=64) |
|||
| Skindex-29 Symptoms | 7.86 | 1.57 (.96-2.18) | <.0001 |
| Skindex-29 Emotions | 10.29 | 2.06 (1.38-2.74) | <.0001 |
A DLQI score change of 5 between consecutive visits is deemed a meaningful change.
In patients with initial CDASI-A scores in the moderate range of (15–26), the change in CDASI-A score needed to achieve such important change in QoL was 5.61 for Symptoms and 7.37 for the Emotions subscale (P <.0001). With initial CDASI-A scores above 26, the change in disease activity needed for this change in Emotion and Symptoms increases (Table 3). If all patients with a baseline score of >14 are evaluated, a 10.27 point change in activity score yields a meaningful change in the Symptoms subscale and a 10.38 point change is needed to achieve meaningful change in the Emotions subscale of the Skindex-29 (Table 3). Changes in CDASI-A scores within the mild range (CDASI-A score ≤14) were insignificant (n=39).
Discussion:
We examined the approximate change in CDASI-A score between two consecutive visits that would lead to a meaningful change in QoL. Literature discussing MCID (minimal clinically important difference) of the DLQI refers to meaningful change as one that demonstrates an improvement in symptoms or improvement in function according to the patient’s point of view.5,12 Previous studies and therapy guidelines have used an MCID of 5 for inflammatory skin diseases.6 We define important or meaningful change equivalently to the MCID, as one that is important in the patient’s perspective. Because approximate changes in the Symptoms and Emotions subscale scores that indicate meaningful change in QoL between two time points have not been established in the past with the creation of the Skindex-29, we derived this value by comparing the Skindex-29 and DLQI relative to the CDASI-A (Table 1). The DLQI and Skindex-29 correlate with each other (Skindex-29 Symptoms r=.6323, Skindex-29 Emotions r=.6744, Skindex-29 Function r=.8598; p <.0001) and are both validated measures of QoL.13 In assessing what change in Skindex-29 scores between visits constitutes one that is important or meaningful, the MCID of 5 for the DLQI was used. In examining the Skindex-29 relative to the DLQI, at least an 8 (7.86)-point change in the Symptoms subscale (P <.0001) and a 10 (10.29)-point change in the Emotions subscale (P <.0001) constituted a minimally important change in QoL in patients with an initial CDASI-A score of >14 (Table 1). Although our study population only included patients with dermatomyositis, the use of an 8-point change in Symptoms and a 10-point change in Emotions in detecting meaningful change in QoL may be applicable to other similar inflammatory connective tissue disorders with prominent cutaneous findings. Patients with CDASI-A scores in the mild disease category were excluded in this analysis, as mild disease activity poorly correlates with the QoL measures of Symptoms and Emotions.4
In DM patients with an initial score of >14, a meaningful change in QoL is apparent at a 32% (P <.0001) change in CDASI-A score for the Symptoms subscale and a 35% (P <.0001) change for the Emotions subscale (Table 2). In patients with moderate to severe disease activity, with an initial CDASI-A score of >14, a 40% change in CDASI-A score can be used as an indicator for a meaningful change in QoL by the Symptoms and Emotions subscales of the Skindex-29. Because a lower percentage change in score is needed to identify meaningful change in both subscales, a 40% change marks a conservative estimate for our recommendation. The use of this threshold CDASI-A score coincides with recent research from our group showing that QoL correlates to CDASI-A scores down to 7 and 10 for Symptoms and Emotions, respectively.4 In Table 2, which shows different groups of CDASI-A scores based on the score at the first visit and percent changes in score needed to result in meaningful change, patients with higher initial activity scores within moderate disease need a smaller percent change to yield an important change in QoL. Based on these results, we recommend the use of a threshold score of greater than 14 when using 40% as an indicator for meaningful change.
When assessing the actual change in CDASI-A score between the first two visits, a change in activity score of 6 in relation to Symptoms (P=.0002) and 7 in relation to Emotions (P=.0007) was needed for a meaningful change in QoL in patients with moderate disease with an initial CDASI-A score between 15 and 26 (Table 3).10 A 6-point and 7-point change in the CDASI-A reflects a meaningful change in QoL, as defined by the Skindex-29, and is also considered clinically significant improvement in disease activity.3 These findings suggest that near or total clearance of skin findings is not absolutely necessary to demonstrate important clinical outcomes for patients in clinical trials, as patients experience a meaningful improvement in their QoL despite retaining some cutaneous activity.
There are a number of limitations to this study. This was a retrospective single-center study in which only patients who completed both the Skindex-29 and the DLQI could be included, along with those who completed at least two visits. Out of the 105 remaining patients, two patients were excluded due to no cutaneous disease activity. Additionally, we did not discriminate patients by the amount of time between their first two visits, which should not impact the objective CDASI-A score.
In conclusion, for those with a threshold CDASI-A score range of >14, a 40% change in CDASI-A score between the first two visits is associated with a meaningful change in QoL. It is important for physicians to know when their treatment of cutaneous disease in patients leads to an important change in their QoL. Although a 20% decrease in CDASI-A score indicates significant clinical improvement, our study demonstrates that a higher percent change in disease activity is needed to achieve an important change in QoL.14 Clinical trials can consider using this 40% change in CDASI-A score as an endpoint when assessing the clinical efficacy of drugs.
What’s already known about this topic?
The Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI) is a validated disease assessment tool used to capture the extent of cutaneous activity and damage.
The Skindex-29 and Dermatology Life Quality Index (DLQI) are standardized and validated measures of quality of life (QoL) for clinical trials and correlate with CDASI activity (CDASI-A) scores.
What does this study add?
Approximate changes in the Skindex-29 that indicate important change (MCID) in QoL in patients between two time points were derived.
We evaluated the change in CDASI-A score between two consecutive visits that would lead to a meaningful change in QoL.
For those with an initial CDASI-A score range of >14, a 40% change in CDASI-A score between the first two visits is associated with a meaningful change in QoL.
What are the clinical implications of the work?
Clinical trials can consider using a 40% change in CDASI-A score as an endpoint when assessing the clinical efficacy of drugs.
Acknowledgments
Funding Statement: This work was supported by National Institute of Health [R21 AR066286] and Veterans Affairs Merit Review [I01BX000706] to VPW.
Footnotes
Disclosures: The University of Pennsylvania owns a copyright of the CDASI.
References:
- 1.Findlay AR, Goyal NA, Mozaffar T. An overview of polymyositis and dermatomyositis. Muscle Nerve 2015; 51: 638–56. [DOI] [PubMed] [Google Scholar]
- 2.Klein RQ, Bangert CA, Costner M et al. Comparison of the reliability and validity of outcome instruments for cutaneous dermatomyositis. Br J Dermatol 2008; 159: 887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson ES, Feng R, Okawa J et al. Improvement in the cutaneous disease activity of patients with dermatomyositis is associated with a better quality of life. Br J Dermatol 2015; 172: 169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaffney R, Tarazi M, Feng R et al. Examining cutaneous disease activity as an outcome measure for clinical trials in dermatomyositis. Journal of the American Academy of Dermatology 2019. [DOI] [PubMed] [Google Scholar]
- 5.Basra MK, Salek MS, Camilleri L et al. Determining the minimal clinically important difference and responsiveness of the Dermatology Life Quality Index (DLQI): further data. Dermatology 2015; 230: 27–33. [DOI] [PubMed] [Google Scholar]
- 6.Basra MK, Fenech R, Gatt RM et al. The Dermatology Life Quality Index 1994–2007: a comprehensive review of validation data and clinical results. Br J Dermatol 2008; 159: 997–1035. [DOI] [PubMed] [Google Scholar]
- 7.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)--a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19: 210–6. [DOI] [PubMed] [Google Scholar]
- 8.Khilji F Clinical meaning of change in Dermatology Life Quality Index scores. Br J Dermatol 2002; 147: 50. [Google Scholar]
- 9.Chren M-M, Lasek RJ, Quinn LM et al. Skindex, a quality-of-life measure for patients with skin disease: reliability, validity, and responsiveness. Journal of Investigative Dermatology 1996; 107. [DOI] [PubMed] [Google Scholar]
- 10.Anyanwu CO, Fiorentino DF, Chung L et al. Validation of the Cutaneous Dermatomyositis Disease Area and Severity Index: characterizing disease severity and assessing responsiveness to clinical change. Br J Dermatol 2015; 173: 969–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goreshi R, Okawa J, Rose M et al. Evaluation of reliability, validity, and responsiveness of the CDASI and the CAT-BM. J Invest Dermatol 2012; 132: 1117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials 1989; 10: 407–15. [DOI] [PubMed] [Google Scholar]
- 13.Goreshi R, Chock M, Foering K et al. Quality of life in dermatomyositis. J Am Acad Dermatol 2011; 65: 1107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chansky PB, Olazagasti JM, Feng R et al. Cutaneous dermatomyositis disease course followed over time using the Cutaneous Dermatomyositis Disease Area and Severity Index. J Am Acad Dermatol 2018; 79: 464–9.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]