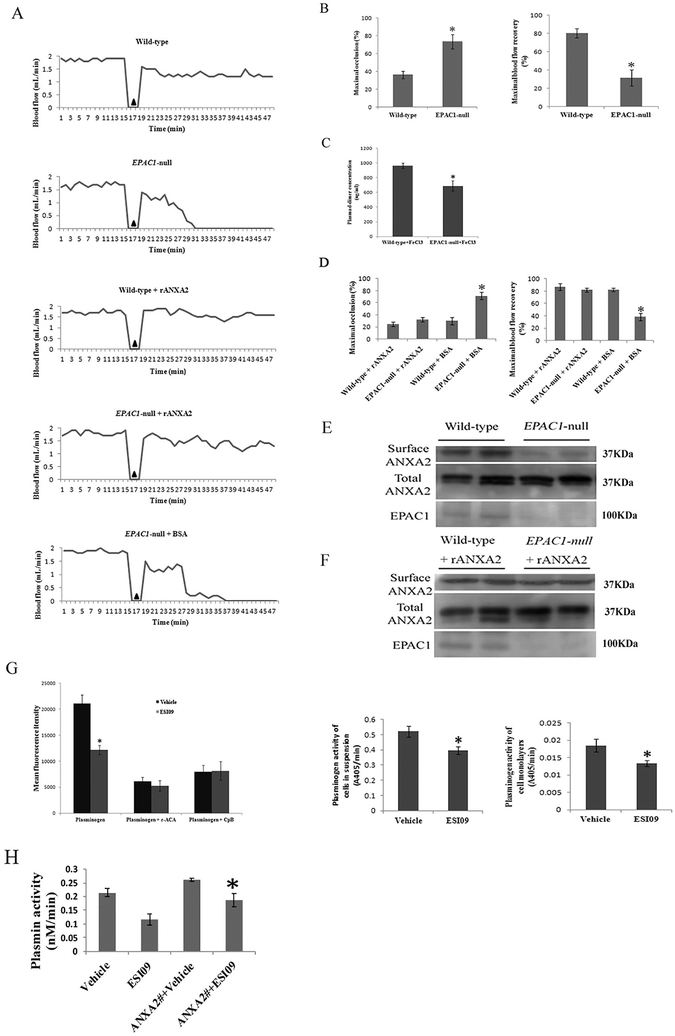
Fig. 3.
Impaired vascular fibrinolytic function can be restored by recombinant ANXA2 in EPAC1-null mice. Representative carotid blood flow before and after three-minute application of 7.5% FeCl3 (▲) (A). Compared to wild-type mice (n = 12), EPAC1-null mice (n = 12) demonstrated a significantly higher maximal occlusion (MaxO, *P < 0.01) and a lower maximal recovery (MaxR, *P < 0.01) (B). Plasma levels of D-dimer were measured after application of 7.5% FeCl3 (C). Compared to wild-type mice (n = 5), EPAC1-null mice (n = 5) demonstrated lower levels of D-dimer (*P < 0.05). Wild-type (n = 4) and EPAC1-null mice (n = 11) were treated with rANXA2, showing no difference in MaxO and MaxR. Compared to EPAC1-null mice pretreated with BSA (n = 5), EPAC1-null mice pretreated with rANXA2 show lower MaxO (*P < 0.01) and higher MaxR (*P < 0.01) (D). WB analysis showed that reduced cell surface expression of ANXA2 in EPAC1-null mice in vivo (E). WB analysis further showed elevated level of aortic endothelial surface ANXA2 in rANXA2-treated EPAC1-null mice after carotid artery exposure to FeCl3, compared to BSA-treated group (F). Cell-based system demonstrated that treatment of ESI09 (n = 8) weakened plasminogen binding to endothelial surfaces and reduced plasmin generation, compared to vehicle-treated group (n = 8) (G) (*P < 0.05). Carboxypeptidase B (CpB) or ɛ-aminocaproic acid (ɛ-ACA) were used as negative controls [8]. HUVECs were transfected with pEGFP-HA vectors expressing full-length ANXA2 before vehicle- or ESI09-treatment. Plasmin activity assay demonstrated that ectopic expression of ANXA2 can attenuate the inhibition of plasmin activity by inactivation of EPAC1 (H) (*P < 0.05).