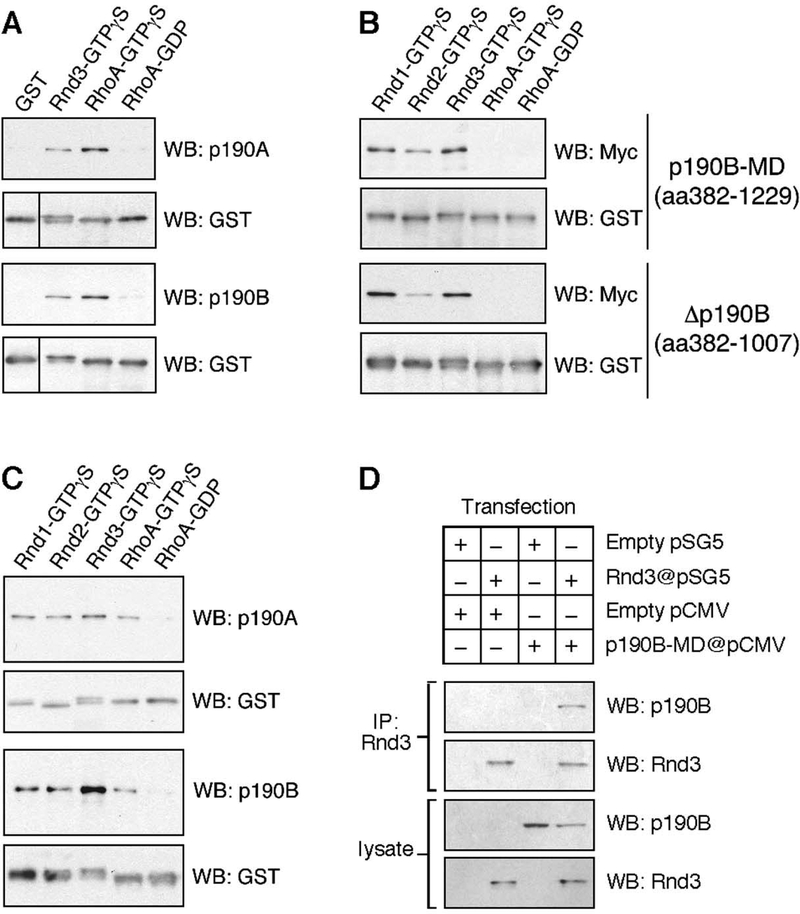
Figure 2: Interaction between Rnd and p190 molecules in GST pull-down and co-immunoprecipitation experiments.
(A) GST pull-down experiments with immobilized GST fusion proteins of Rnd3 loaded with GTPγS, or RhoA loaded with GTPγS or GDP, as well as GST alone demonstrates an interaction between Rnd3 and endogenous p190A or p190B. In the western blot to detect GST fusion proteins, the left lane has been shifted upwards to compensate for the higher mobility of non-fusion GST.
(B) GST pull-down experiment from Cos7 cells transfected with expression constructs encoding Myc-epitope tagged p190B-MD (amino acids 382–1229), or a Myc-tagged fragment corresponding to amino acids 382–1007 of p190B. As shown, GTPγS-loaded Rnd1, Rnd2, and Rnd3, but not RhoA, bind p190B-MD or the 382–1007 fragment.
(C) GST pull-down experiment with insect cell purified p190A and p190B demonstrating that the interaction between Rnd proteins and p190 molecules is direct.
(D) Co-immunoprecipitation of p190B-MD with Rnd3 from lysates of Cos7 cells co-transfected with p190B-MD and Rnd3 expression vectors.