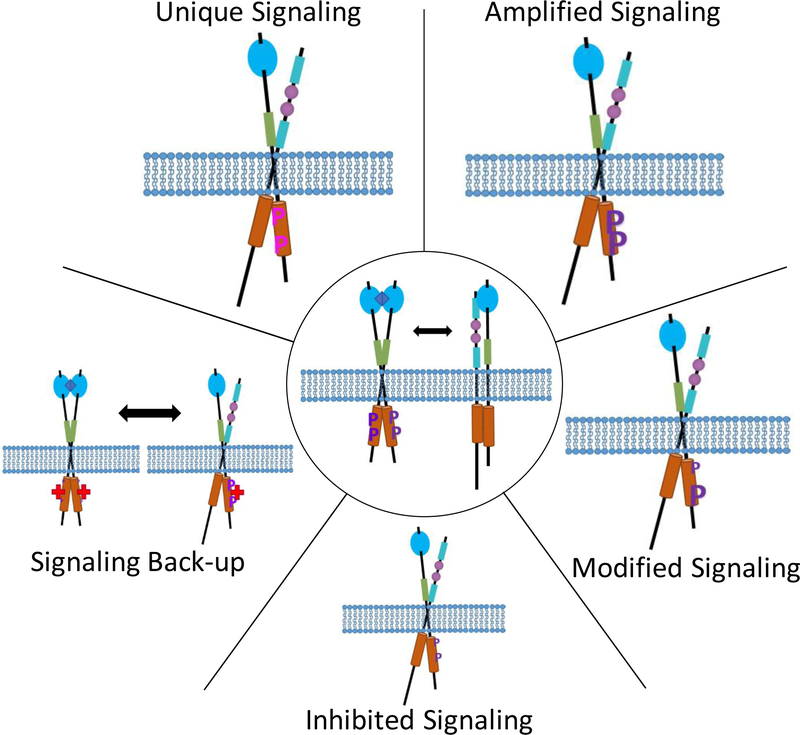
Figure 5.
Depiction of the possible effects of RTK heterodimerization. Center shows a generic, activated RTK homodimer in equilibrium with an RTK heterodimer (blue diamond, ligand and purple P, phosphorylation). Starting from the top left and going clockwise, unique signaling, where the heterodimer causes signaling not seen in the homodimers, possible due to unique tyrosines being phosphorylated (pink P). Amplified signaling, where the heterodimer has a stronger downstream signal than the homodimer, possible due to increased phosphorylation or decreased degradation. Modified signaling, where the heterodimer has a different probability of phosphorylation or adaptor protein binding than the homodimer, and it is possible that some tyrosines have increased phosphorylation while other tyrosines have decreased phosphorylation. Inhibited signaling, where the heterodimer has a weaker downstream signal than the homodimer, possible due to the heterodimer being inactive or recruitment of a molecule which directly dephosphorylates the RTK. Signaling back-up, where the homodimer has been inhibited, possibly by a drug (red cross), but signaling can continue due to direct phosphorylation by the heterodimerization partner. Note that the heterodimers are shown as unliganded, which may not always be the case (i.e., some heterodimers could be liganded), and that the heterodimer effects are shown as only affecting signaling of one of the RTK species, although the effects could be bidirectional (i.e., the signaling of both RTK species could be affected).