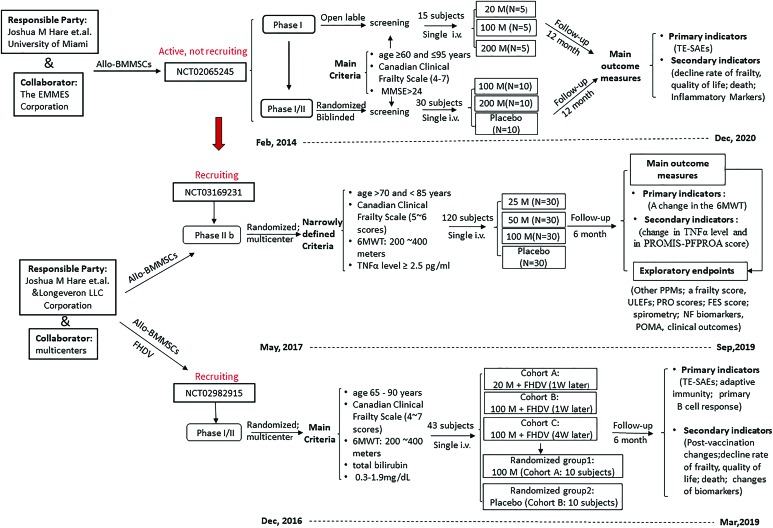
FIG. 2.
Current worldwide ongoing clinical trials of stem cells for frailty. This flowchart shows the main schemed work of the three total ongoing clinical trials (NCT01501461withdrawn); the NCT03169231 is the next-step trial of NCT02065245, mainly to assess the safety and efficacy of Longeveron Mesenchymal Stem Cells, with more narrowly defined criteria. Allo-BMMSCs, allogenic bone marrow mesenchymal stem cells; FES, falls efficacy scale score; FHDV, fluzone high dose vaccine; M, million cells; 6MWT, 6-minute walk distance test; MMSE, Mini-Mental State Examination; N, number of patient; NF, neuroinflammatory biomarkers; POMA, Performance Oriented Mobility Assessment; PPMs, physical performance measures; PROMIS-PFPROA score, PROMIS-Physical Function Patient Reported Outcome Assessment; PRO scores, patient-report outcome scores; Single i.v., single peripheral intravenous infusion; TNF-α, tumor necrosis factor-α; ULEFs, upper and lower extremity function; W, week. It is originally produced on basis of data from clinicaltrials.gov. Color images are available online.