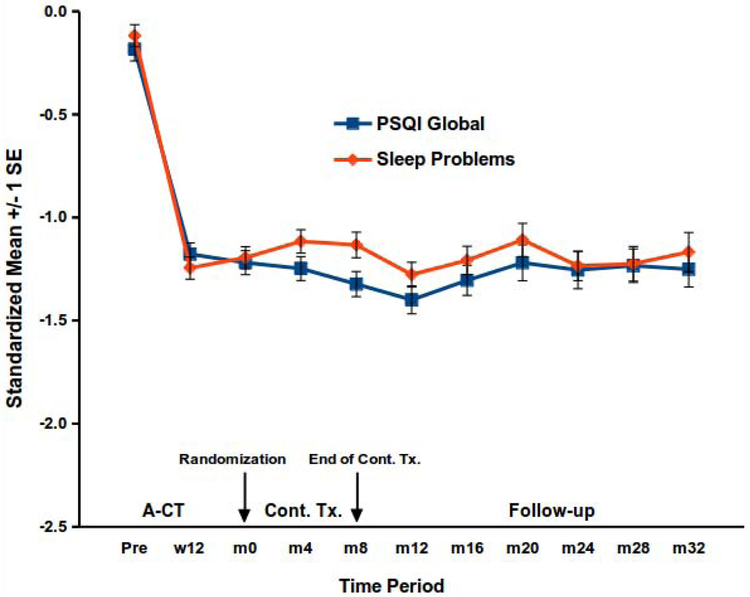
Figure 2.
Changes in sleep disturbance among higher risk A-CT responders.
PSQI = Pittsburgh Sleep Quality Index. A-CT = Acute-phase cognitive therapy. Cont. Tx. = continuation treatment. w = week, m = month. Among higher risk responders to A-CT, additional changes in the PSQI global score and sleep-problems scale were not statistically significant through 8 months of continuation treatment (Cont. Tx; continuation cognitive therapy, fluoxetine with clinical management, or pill placebo with clinical management) or through 32 total months of follow-up.