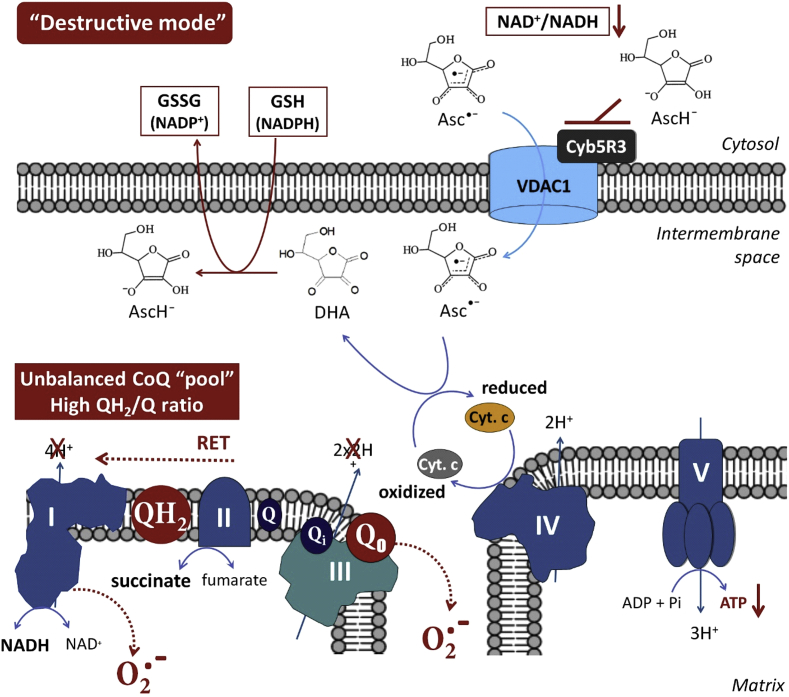
Fig. 4.
“Destructive mode” of action of ascorbate free radical via the OMM Cyb5R3/VDAC1 at high (“therapeutic”) concentrations of vitamin C in cancer cells (at normoxia).
High intracellular concentration of ascorbate may induce Cyb5R3 “end-product inhibition”, accompanied by elevated levels of ascorbate free radical (AFR; Asc•) in the mitochondrial intermembrane space and decreasing of NAD+/NADH ratio in the cytosol. AFR may transfer one electron to oxidized cytochrome c, causing a partial (or complete) arrest of electron flow between Complex-III and Complex-IV. Rapid reduction of cytochrome c by AFR effectively competes with and perturbs the proton pumping at Complex-III, as well as the temporal electron transport provided by CoQ cycles of Complex-III. Precisely timed redox sequences responsible for the efficient flow of electrons from Complex-III to oxidized cytochrome c are vulnerable to AFR flux. This drives the CoQ “pool” to a more reduced (overcharged) form. This also causes the lifetime of semi-ubiquinone in the Qo pocket to lengthen, allowing oxygen to accept the second electron from ubiquinol during the second step of the CoQ cycle, yielding superoxide. This can interfere with the timing of the Qo/Qi cycle and consequently the CoQ “pool” redox balance, impairing mitochondrial respiration. A highly reduced CoQ “pool” inhibits proton pumping yielding up to 80% lower ATP plus higher levels of superoxide. When Complex-III is blocked, succinate (from the citric acid cycle) builds up, mitochondrial membrane potential rises, and the CoQ “pool” becomes unbalanced. Reverse electron transport (RET) to Complex-I is driven, which causes a superoxide “burst” [59,60]. RET is also accompanied by synthesis of succinate and NADH. We propose that the “destructive mode” caused by high-dose vitamin C may contribute to RET not only during tissue reperfusion at angiogenesis, but also during tumor hypoxia and normoxia in cancer cells.
In addition, the reduction of cytochrome c by AFR results in production of DHA. DHA is converted to ascorbate by glutathione, which provokes a depletion of reducing equivalents in cancer cells – a crucial factor for their survival.
VDAC1 – voltage dependent anion channel 1; Cyb5R3 – NADH:ferricytochrome b5 oxidoreductase 3; OMM – outer mitochondrial membrane; Cyt. c – cytochrome c; RET – reverse electron transport; DHA – dehydroascorbate; AcsH− – ascorbate in anion form; Asc• – ascorbate free radical.