Figure 4.
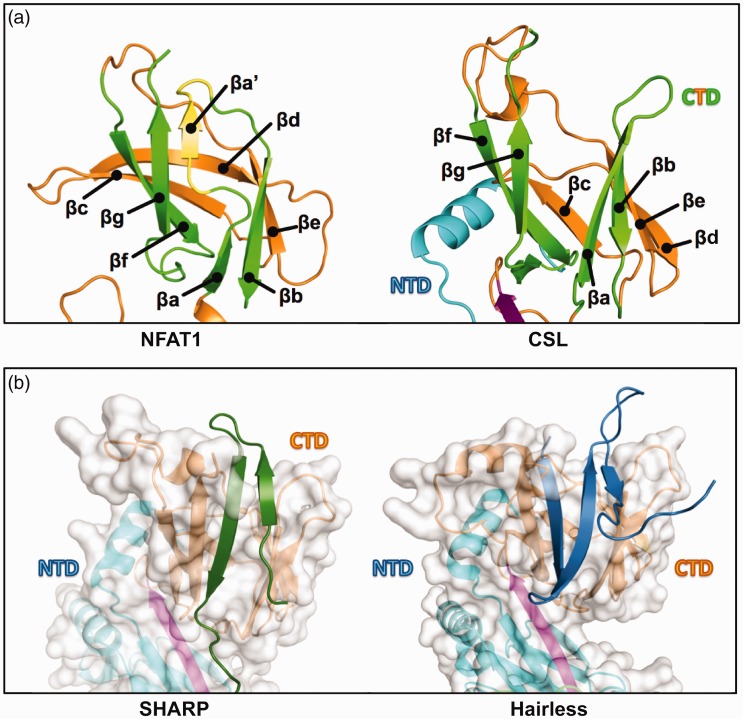
A second CSL binding pocket. (a) Canonical immunoglobulin (Ig) domain vs. CSL C-terminal domain (CTD) immunoglobulin fold. Left, The NFAT1 C-terminal Rel homology region (RHR-C) contains a canonical Ig fold with connected but discrete βa and βaʹ strands that form β sheet interactions with, respectively, βb and βg strands (strands are colored green for comparison, with βaʹ alone highlighted in yellow); PDB: 1S9K. Right, CSL lacks βaʹ and instead has an extended βa strand that interacts with βb but is separated from βg; PDB: 2FO1. (b) Left, the separation of βa and βg in CSL creates a protein binding cleft that SHARP (dark green) occupies for partial CSL binding. Out-of-view, SHARP extends C-terminally to bind the BTD; PDB: 6DKS. Right, Hairless (blue) also binds to the CTD cleft, but binding requires a larger conformation change to bury more protein mass; PDB: 5E24. This leads a dramatic increase in affinity (1 nM) compared to the SHARP-CTD interaction (60 µM).