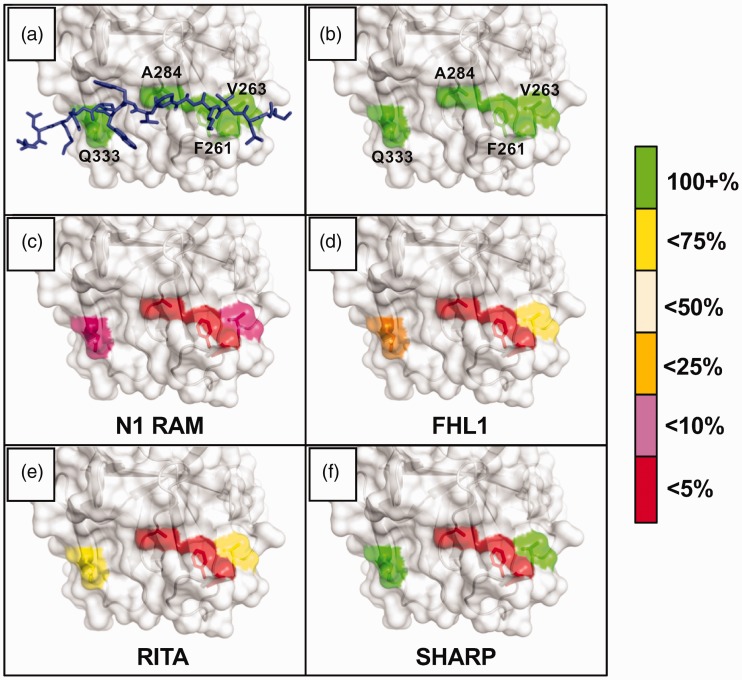
Figure 5.
Notch coregulator binding to CSL mutants. The figure graphically depicts the results of rigorous isothermal titration calorimetry (ITC) experiments involving series of CSL BTD mutants and their effects on binding between CSL and established coregulators. The BTD residues selected for mutation—F261, V263, A284, and Q333—were chosen to disrupt RAM binding without dramatically altering CSL stability. They are shown with (a) and without (b) an overlay of the FHL1 peptide; PDB: 4J2X. The colors highlighting the individual residues reflect the binding retained after mutation, i.e. if a mutation caused the Kd to increase more than 4-fold but less than 10-fold (10–25% binding retained), the residue would be colored orange. (c) and (d) Notch1 RAM and FHL1 were tested against the arginine mutants: F261R, V263R, A284R, Q333R. Drastic decreases in binding were seen for both coregulators with the F261 and A284 mutants, illustrating the importance of the platform created by the phenylalanine sidechain, as well as the cleft afforded by the compact alanine sidechain. (e) and (f) RITA and SHARP were tested against the alanine/valine mutants: F261A, V263A, A284V, Q333A. Later experiments moved to these mutants in order to avoid introducing artifacts due to the lengthy and charged arginine sidechains.