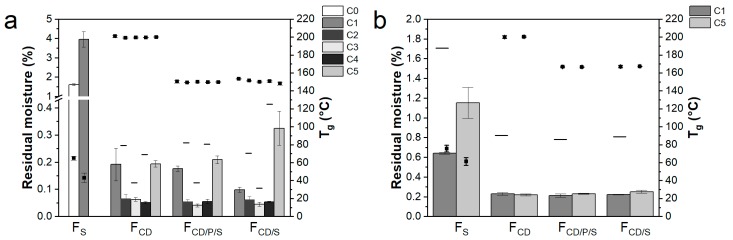
Figure 5.
Residual moisture and Tg. Data is given for (a) 10 mg/mL mAb formulations and (b) 50 mg/mL mAb formulations for different formulations and freeze-drying cycle conditions. Residual moisture levels are shown as bars and lines. Lines show residual moisture level of vials stoppered after primary drying. Squares show Tg values of the lyophilisates. Values are means (n = 3) ± standard deviation.