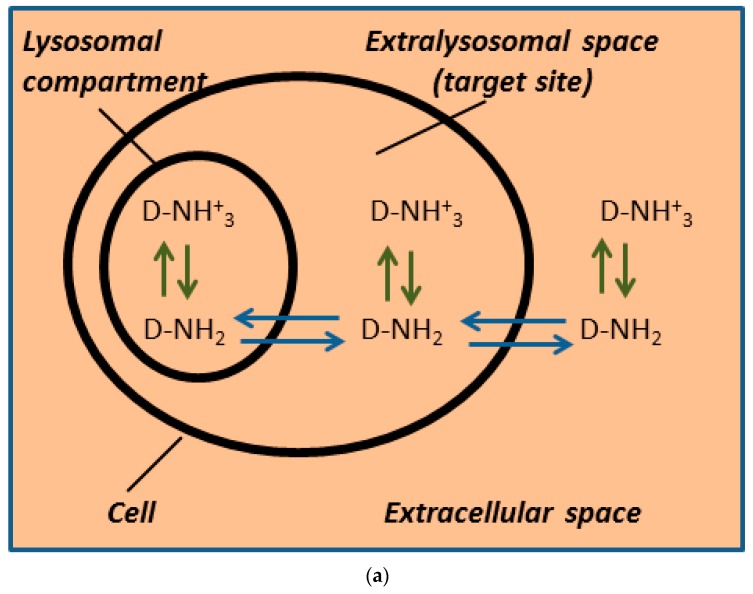
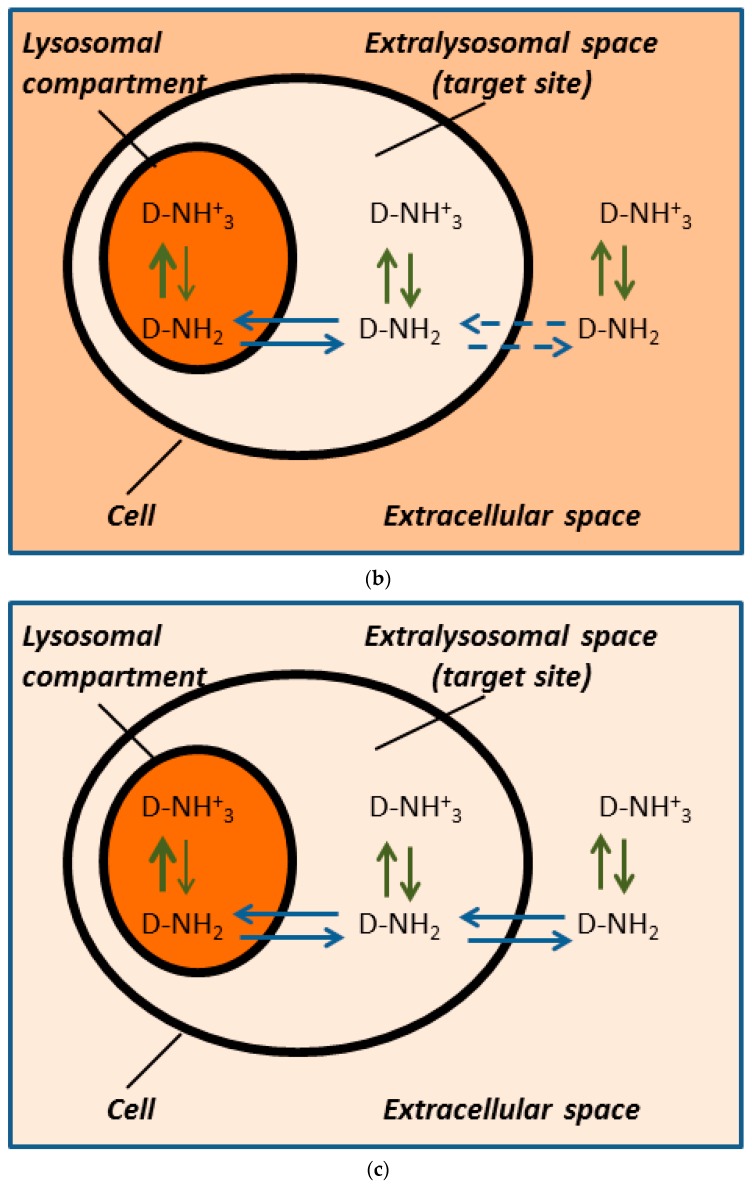
Figure 1.
Weak-base drug distribution within the cell in vitro. Our model includes several theoretical assumptions: (i) uncharged forms of weakly basic drugs can freely diffuse across cellular membranes [5,20]; (ii) only two interactions are considered, the Henderson–Hasselbach equilibrium ↑ ↓ and the passive diffusion of uncharged molecules ↔; (iii) the extra and intracellular (extralysosomal space) pHs are equal. Color saturation corresponds to the drug concentration. (a) Drug distribution without lysosomal sequestration. (This may occur, for example, after the addition of BafA1, a vacuolar ATPase inhibitor.) Uncharged molecules can freely diffuse across cell membranes. (b) Drug distribution with lysosomal sequestration. Uncharged molecules can freely diffuse only across the lysosomal membrane. Under such conditions, the lysosomal sequestration of a drug can dramatically reduce its extralysosmal concentration (drug concentration at target sites), and thus mediate drug resistance. However, such a model is not applicable since it does not fulfil theoretical assumptions. In fact, the diffusion of uncharged molecules across the plasma membrane would dissipate the gradient between extra and intracellular space. (c) Drug distribution with lysosomal sequestration. Uncharged molecules can freely diffuse across cell membranes. The lysosomal sequestration of a drug that reduces drug concentration at target sites (= in extralysosomal space) and simultaneously decreases the extracellular drug level can mediate resistance to this drug. This is the only model to fulfil theoretical assumptions.