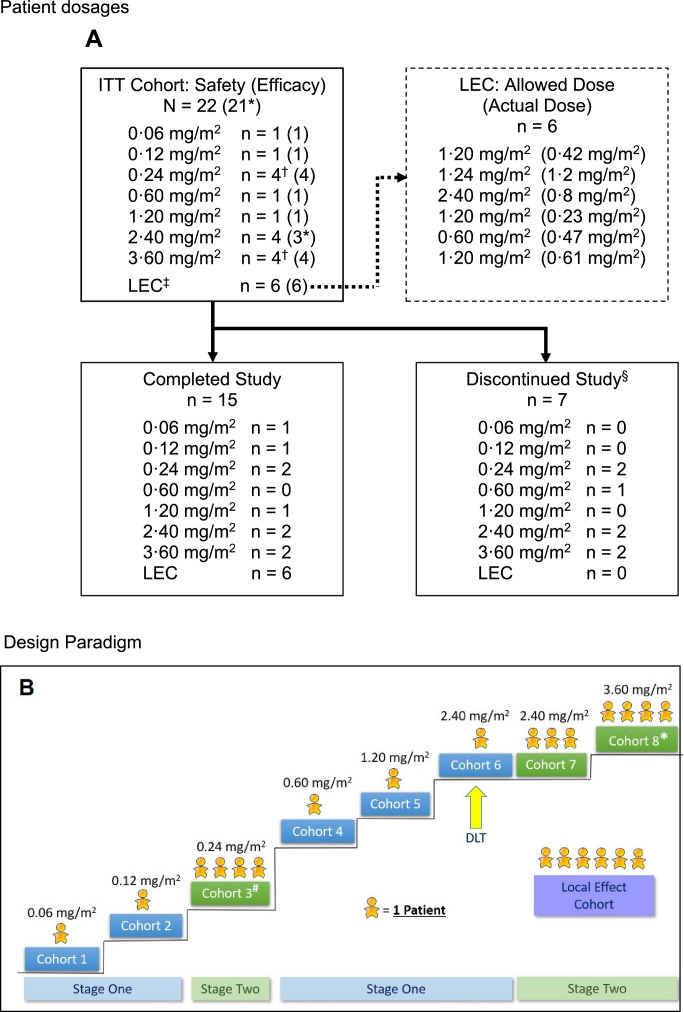
Fig. 1.
A: Patient dosages.
EC = local effect cohort; RECIST = Response Evaluation Criteria in Solid Tumors.
*One patient at dose level 2·40 mg/m2 was excluded from the efficacy population because a post-baseline RECIST assessment of the target tumors was not performed.
†In these cohorts, one patient experienced leakage of tigilanol tiglate, so an additional patient was added to the cohort.
‡Six patients had insufficient tumor mass to be enrolled in the dose- escalation stages and were enrolled in the LEC. These patients received the highest concentration of tigilanol tiglate that had been shown to be tolerated, a total dose no higher than the dose tested in the most recent dose-escalation cohort, and a minimum volume of 0·1 mL per tumor. This cohort followed the same assessment schedule as the dose-escalation cohorts.
§Six patients discontinued participation due to disease progression and one due to unintentional lack of follow-up. The mean time to discontinuation was 25·4 days (range: 18 to 43 days).
B: Design Paradigm.
# A patient in this cohort experienced leakage following dosing, therefore a total of four were enrolled to satisfy dose escalation rules
* A patient in this cohort was under-dosed, therefore a total of four were enrolled to satisfy dose escalation rules.