Figure 1.
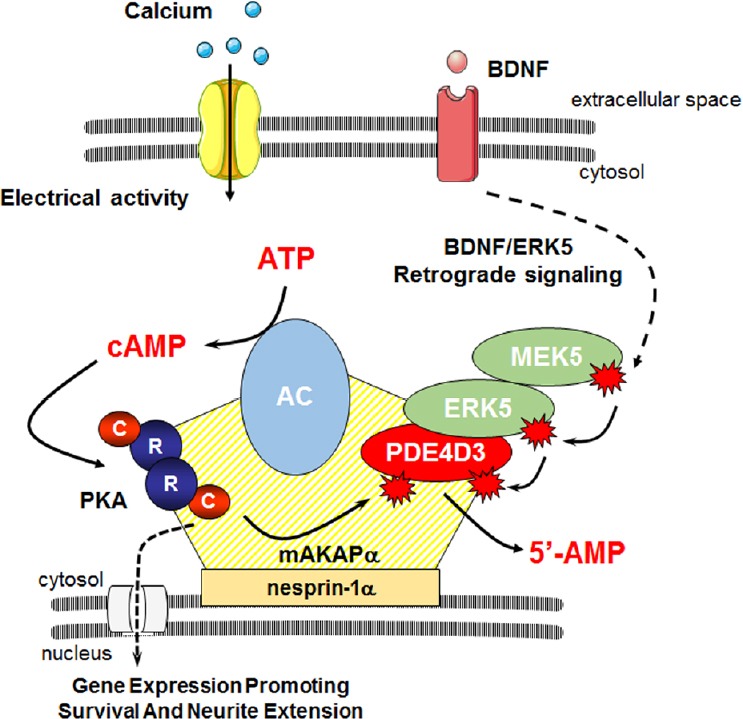
Model of cAMP signaling regulation in the perinuclear compartment.
Neuronal membrane depolarization promotes calcium entry and activation of calcium-sensitive adenylyl cyclases (ACs) leading to increased intracellular cAMP concentration. cAMP binds tetrameric protein kinase A (PKA) and results in dissociation of regulatory (R) and catalytic (C) subunits. At mAKAPα signalosomes, PKA may then phosphorylate phosphodiesterase 4D3 increasing its cAMP hydrolytic activity, thus providing a negative feedback loop by which PKA controls its own activity. mAKAPα also may provide a platform for integration of signals induced by neurotrophins such as brain-derived neurotrophic factor (BDNF). In response to BDNF, mitogen-activated protein kinase kinase 5 (MEK5) activates extracellular signal-regulated kinase 5 (ERK5), which phosphorylates PDE4D3 inhibiting cAMP hydrolysis. This neurotrophin-dependent mechanism regulating PDE4D3 activity may allow for increased local cAMP and induction of genes involved in neuronal growth and survival. cAMP: Cyclic adenosine monophosphate.
