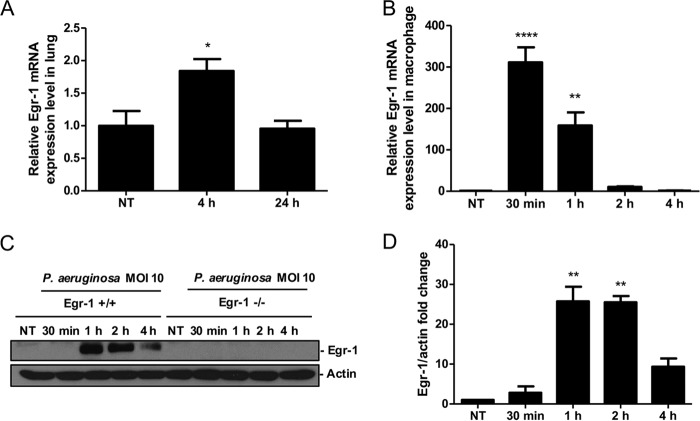
FIG 1.
Egr-1 expression is induced in response to P. aeruginosa infection both in vivo and in vitro. Wild-type (+/+) and Egr-1-deficient (−/−) mice were intranasally infected with 1 × 109 CFU/mouse of P. aeruginosa 8821 for 4 h or 24 h or with an equivalent volume of saline solution as a control (NT). The total RNA extracted from lungs was reverse transcribed to cDNA and subjected to real-time quantitative PCR for Egr-1 gene expression. The gene expression was normalized to the HPRT housekeeping control gene (A) (n = 3 ± SEM; *, P < 0.05). BMMs were infected with P. aeruginosa strain 8821 at an MOI of 10 for 30 min, 1 h, 2 h, or 4 h or left untreated (NT). Total RNA isolated from these cells was reverse transcribed to cDNA and subjected to real-time quantitative PCR for Egr-1 gene expression. The Egr-1 mRNA levels were normalized to endogenous control HPRT (B) (n = 3 ± SEM; **, P < 0.01; ****, P < 0.0001). Cell lysates were subjected to Western blotting for Egr-1 protein expression, and actin was used as a loading control. Blots are representative of three independent experiments (C). Densitometry analysis of Egr-1 protein levels was normalized to actin, and data are presented as fold change (D) (n = 3 ± SEM; **, P < 0.01).