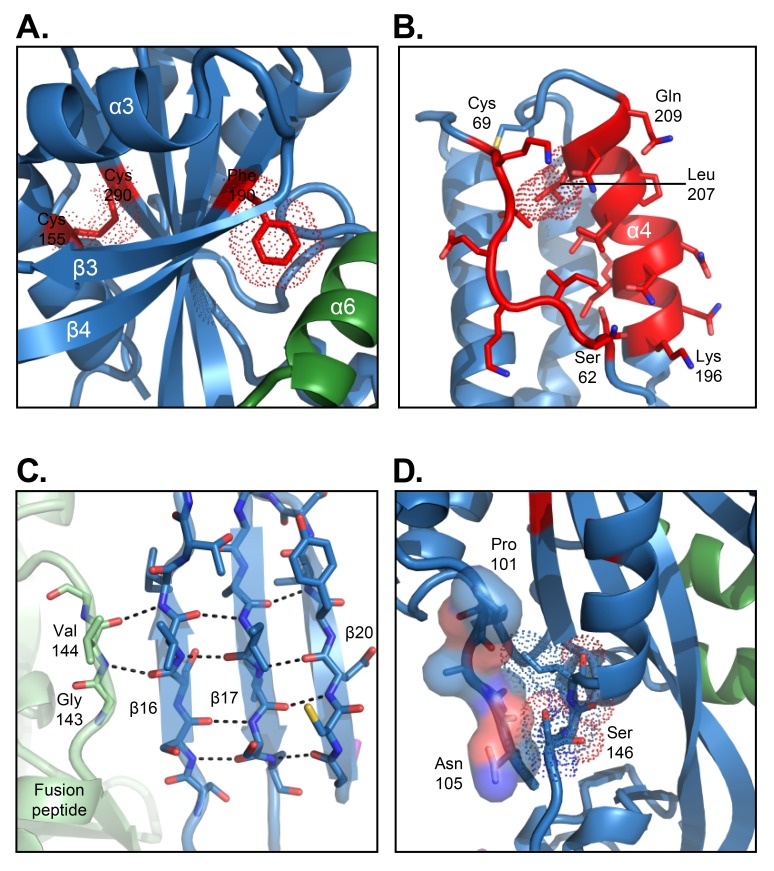
Figure 2.
Atomic-level details of the RSV B18537 F glycoprotein structure. (A) Prefusion stabilizing DS-Cav1 mutations (S155C, S190F, S290C) are shown with van der Waals surface (red dots) with the adjacent secondary structure including the domain III β-propeller fold shown in ribbon format. (B) Antigenic site Ø located at the apex of each protomer is highlighted in red with side chains in stick representation. The V207L mutation, which is part of the stabilizing mutations, is highlighted in dot representation. (C) Inter-protomer hydrogen bonds between the fusion peptide of one protomer and β strands 16, 17, and 20 from an adjacent protomer are indicated by dotted lines. (D) Interaction between the F2 C-terminus and the fusion peptide within a single protomer. The F2 C-terminus is shown in surface representation and the fusion peptide shown with dotted van der Waals surface.