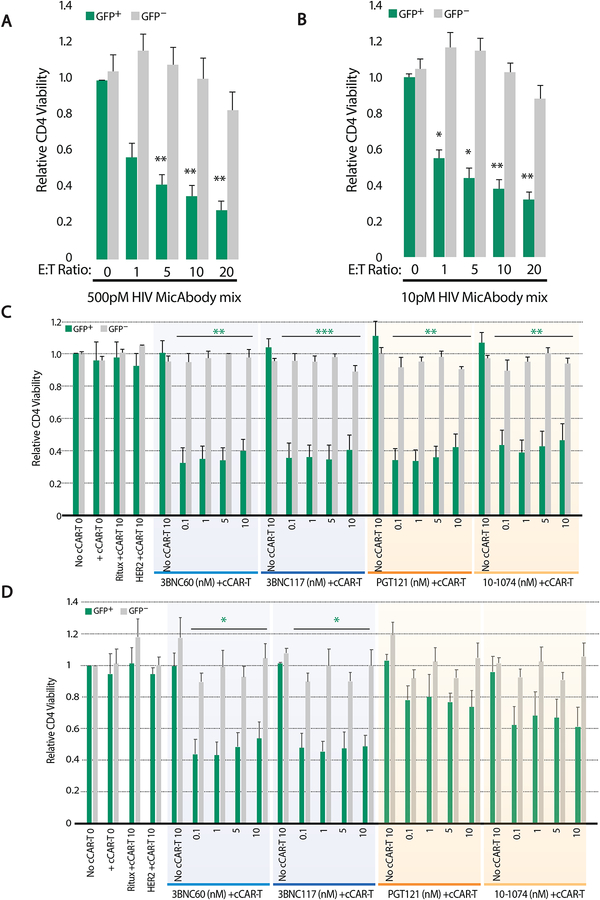
Figure 2: Specific killing of primary CD4 T cells infected with CCR5-tropic HIV by convertibleCAR-T combined with HIV Env-specific MicAbodies.
To determine the optimal effector to target cell ratio, one million tonsil derived cells (~1×104 HIV/GFP-infected target cells) were incubated with a range of cCAR-T effector cells from zero (0:1) to 2×105 (20:1) cCAR-T cells: target cells for 48 hours with the mix of four HIV Env-specific MicAbodies (Mix). In the absence of cCAR-T cells (0:1), the donor-matched untransduced CD8 T cells were present. GFP+ live GFP+/CD3+/CD8− cells were counted to assess reduction in target cells (GFP+) and live GFP−/CD3+/CD8− cells were counted to assess off target killing. HIV mix concentration was tested with high (0.5nM) (A) or low (10pM) (B) concentration of each individual MicAbody in the HIV MicAbody mix. Data derived from three independent experiments; mean + SEM. (C) Specific killing of R5 tropic HIV-1 (BaL) infected cells or (D) X4 tropic HIV-1 (NL4–3) used to infect tonsil cells followed by testing of individual HIV-specific MicAbody for arming of cCAR-T cells. One million tonsil derived cells (~1×104 infected cells) were incubated with 1×105 CAR-T cells for 48 hours, in the presence of different concentrations (0.1–10nM) of HIV Env-specific MicAbodies. B-cell specific MicAbody (Ritux) and anti-HER2 specific MicAbody (HER2) were used as negative control MicAbodies. Results are presented relative to the no cCAR-T control. For each individual MicAbody, an internal control of no cCAR-T supplemented with the highest MicAbody concentration tested is presented. To assess off target killing or generalized in-well toxicity, viability of GFP− CD4 T cells was assessed. This experiment was performed four times using cells from independent donors. Data are represented as mean + SEM. * = p≤0.05, ** = p≤0.01, *** = p≤0.001 (compared to no cCAR-T presence). See also Figures S1–3 and S5.