Table 1.
Key features of current bioengineered platforms, in vitro and ex vivo, developed for chronic wound infection studies.
| Platform | Components | Platforms and their key features | References |
|---|---|---|---|
| In vitro |
Microbes+Host Cells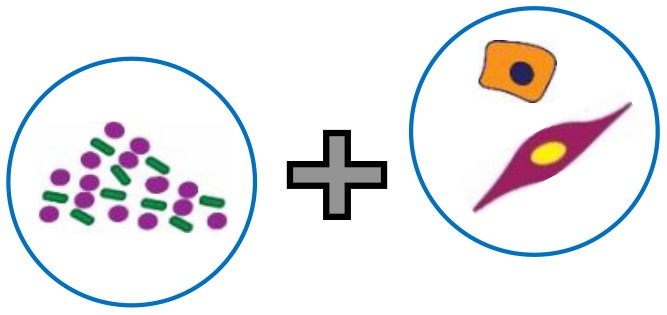
|
Human Skin cells with biofilm or biofilm-conditioned media
Study the effects of wound colonizing bacteria by co-culturing human skin cells such as keratinocytes and fibroblasts with biofilms. It recapitulates host-microbe interactions in the wound bed resulting in changes in host cell migration, proliferation, and gene expression. Human Skin Equivalents (HSEs) 3D structures that mimic human skin layers and recapitulate bacterial attachment and biofilm formation under conditions close to native architecture. |
Holland et al., 2008, 2009; Charles et al., 2009; Kirker et al., 2009, 2012; Secor et al., 2011; Haisma et al., 2013; Tankersley et al., 2014; Alves et al., 2018 |
Microbes+Immune Cells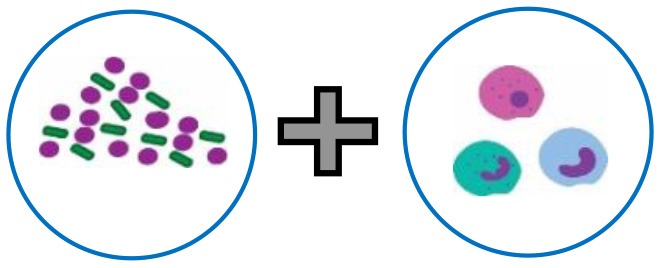
|
Infection-immunity interface on a microfluidic platform
Study interactions between the wound pathogen S. aureus (not specific for biofilms) and neutrophils across two compartments, enabling the study of neutrophil recruitment, migration, and engulfment. |
Brackman and Coenye, 2016 | |
Microbes+Extracellular Matrix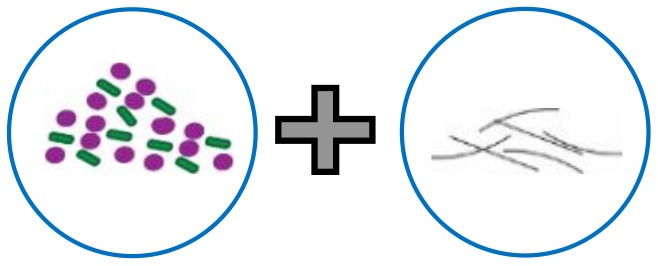
|
Polymer surface coated with gel-like collagen matrix
Study the role of matrix in biofilm formation and structure using comparisons between coated and uncoated surfaces. Collagen mold model with transwell inserts Biofilms embedded in collagen and structured as a void, recapitulating biomimetic effects such as antibiotic diffusion distance through the matrix. |
Werthén et al., 2010; Price et al., 2016 | |
Microbes+Wound fluid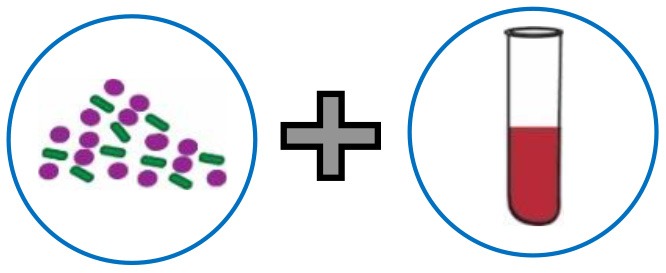
|
Lubbock model (Bolton broth) and its variants
Widely-used to mimic the wound infection state. It enables the study of biofilms and interspecies interactions and has been used to study the effects of antibiotics and other antimicrobial compounds on biofilms. Simulated sweat and serum media Enables the study of growth and biofilm formation under wound-relevant nutritional and chemical conditions. |
Sun et al., 2008, 2014; Dalton et al., 2011; DeLeon et al., 2014; Dowd et al., 2014; Sojka et al., 2016 | |
| Ex vivo |  |
Biological skin tissue from pigs: A high degree of anatomic and physiological similarity to human skin and immune system. Enables the actual creation of a wound (thermal injuries, infected state). Biological tissue supports biofilm growth. Enables testing of immune parameters such as cytokine responses. Can be leveraged to test therapeutics under closely human-relevant conditions. |
Steinstraesser et al., 2010; Yang et al., 2013; Thet et al., 2016 |
| Porcine skin | |||
 |
Biological tissue from human skin:
Can faithfully recapitulate biomimetic features of the chronic wound infection state. Demonstration of biofilm formation and critical host immune factors including cellular and cytokine responses. Can be leveraged to test therapeutics under human-relevant conditions. |
Misic et al., 2014; Schaudinn et al., 2017; Ashrafi et al., 2018 | |
| Human skin |
