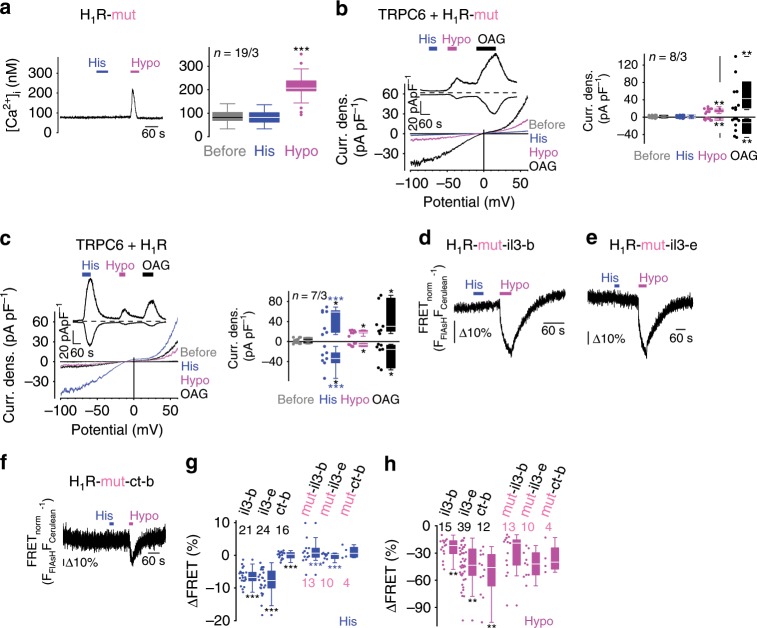
Fig. 3. Mechanical H1R activation is independent of agonist binding.
a Calcium imaging of fura-2 loaded HEK293 cells overexpressing H1R with two amino acid exchanges (D116A and F433A; H1R-mut) resulting in disruption of histamine binding. Representative trace of [Ca2+]i with applications of 100 µM histamine (His) and of hypoosmotic solution (Hypo, 150 mOsm kg-1) and summary of [Ca2+]i are displayed. ***P < 0.001; Wilcoxon matched-pairs signed-rank test to compare to basal [Ca2+]i (‘before’). b, c Whole-cell measurements of HEK293 cells co-expressing TRPC6 and the H1R-mutant (b) or wild-type H1R (c). Representative current density (Curr. dens.)-voltage curves (left) and current density-time courses (insets) with application of 100 µM histamine (His), hypoosmotic bath solution with 250 mOsm kg-1 (Hypo) and of the TRPC6 activator 1-Oleoyl-2-acetyl-glycerol (OAG, 100 µM). Summaries of Current densities at holding potentials of ±60 mV before and during application of histamine, hypoosmotic bath solution and OAG (right). *P < 0.05, **P < 0.01, black asterisks; Wilcoxon matched-pairs signed-rank test to compare to basal current densities (‘before’) and ***P < 0.001, blue asterisks; Mann–Whitney U test to compare wild-type H1R and H1R-mutant. d–f Representative FRET measurements of indicated FRET constructs with impaired histamine binding. g, h Summaries of FRET signal changes induced by application of histamine (g) and of hypoosmotic solution (h). ***P < 0.001, blue asterisks; Mann–Whitney U test compared to wild-type and H1R-mutant FRET constructs and **P < 0.01, ***P < 0.001, black asterisks; Kruskal–Wallis test to compare all wild-type and H1R-mutant FRET constructs. a–c n = x/y indicates the sample size, where x is the number of measured cells and y is the number of coverslips from at least 3 experimental days. a–c, g, h Data are displayed as boxplots (median plus interquartile range (IQR) and whisker (max. 1.5-fold IQR)). See also Supplementary Figs. 2, 3. Source data are provided as a Source Data file.