Abstract
Lauric acid (LA) has a broad spectrum of anti-microbiological activities against enveloped viruses and various bacteria, and might be useful to protect against microbial infection and control the balance and distribution of bacteria in human gut microbiota. It is not necessarily more difficult to measure antimicrobial activity the traditional way, but it is, however, more laborious. In the present study, we developed a new method to measure the antimicrobial activity of LA in multiple samples with a microplate reader. A “test complex” (TC) was produced consisting of 100 μL of agar medium with LA in the bottom layer and 300 μL of broth in the top layer in 96-well deep-well microplates. Afterward, analysis of the broth in the top layer showed that the antimicrobial activity was the same as that of the “control complex,” (CC) which consisted of 100 μL of agar medium in the bottom layer and 300 μL of broth with LA in the top layer. Furthermore, evaluation of the antimicrobial effect of the TC when using a microplate reader was the same as that with the use of the colony counting method. The colony counting method has confirmed that the antimicrobial activity of LA when bacteria are inoculated into the broth was equivalent between CC and TC, and we validated this by correlating the number of bacteria with absorbance. In addition, the broth itself in TC was transparent enough that the turbidity of broth can be used as an index of the number of bacteria, which enabled the use of a microplate reader for multiple samples. For human gut microbes, LA was shown to have low antimicrobial activity against commensal lactic acid bacteria, but high antimicrobial activity against pathogenic Bacteroides and Clostridium, suggesting that LA might modulate intestinal health, as confirmed by the proposed method.
Keywords: screening, human gut microbiome, antimicrobial activity, lauric acid (LA), antimicrobial method
Introduction
Fatty acids (FAs) form long hydrocarbon chains capped by carboxyl groups and have unbranched chains of 4 to 28 carbon atoms, which are either saturated or unsaturated. Some medium-chain FAs (MCFAs), such as lauric acid (LA) and caprylic acid (CA), have a broad spectrum of anti-microbiological activities against enveloped viruses and various bacteria in vitro1–5. Several studies have suggested that some MCFAs disrupt the bacterial cell wall or membrane to protect host cells against infection6,7. FAs, as well as sphingolipids, are involved in the physical, permeability, and immunologic barrier functions of the skin and mucosa5,8,9, and it is predicted that the antimicrobial activities of MCFAs contribute to these protective functions10,11. Furthermore, previous studies have shown that MCFAs added to the diet have a protective effect against bacterial invasion of the mucus3.
In general, in vitro antimicrobial analysis of MCFAs involves a bacterial colony counting method whereby bacteria are inoculated into broth medium containing the indicated concentrations of MCFAs and incubated at 37°C. At the specific time points, the bacterial suspensions are serially diluted by 10-fold and each dilution is plated on an agar plate, which is incubated for a set time, and then the bacterial colonies on the plate are counted. However, this method requires a great number of agar medium plates, and the procedure is rather complicated and requires a relatively long time to complete. Furthermore, with traditional methods, there is a risk that operations for dilutions many times may cause large numerical errors. Therefore, it is difficult to measure and compare the antibacterial activities of many kinds of MCFAs against various types of bacteria in a timely manner and at a higher throughput.
Since the number of bacteria in broth medium is correlated with the turbidity of the media12, a simple measurement of turbidity with a microplate reader could be used to calculate the number of bacteria. However, the presence of FAs muddies the broth medium, making it impossible to precisely calculate the number of bacteria.
Several studies have reported that the antibacterial activities of antimicrobial hydrophilic materials can be measured with the disc diffusion antimicrobial test13–16, where the materials penetrate the agar medium to prevent bacterial growth, resulting in the formation of clear zones around each disc that indicate the inability of the test organism to survive in the presence of an antibiotic. Recently, we confirmed that some MCFAs create a clear zone in the disc diffusion antimicrobial test, suggesting that the hydrophilic part of FAs in the medium is able to penetrate the agar and to inhibit bacterial growth. However, it is difficult to quantitatively evaluate the antimicrobial effect of the test samples with the disk diffusion test.
Therefore, we designed an assay where the FAs are encapsulated by the agar medium and the hydrophilic part of the FA diffuses into the broth medium to inhibit bacterial growth (Fig. 1). Then, we assumed that it would be possible to quickly and conveniently measure the antimicrobial effect of FAs using a microplate reader to measure turbidity. The results of the present study showed that it is possible to evaluate the antimicrobial activity of a large quantity of FAs with our proposed method. Furthermore, we applied this method to evaluate the antimicrobial activity of LA against human pathogens and human gut microbes.
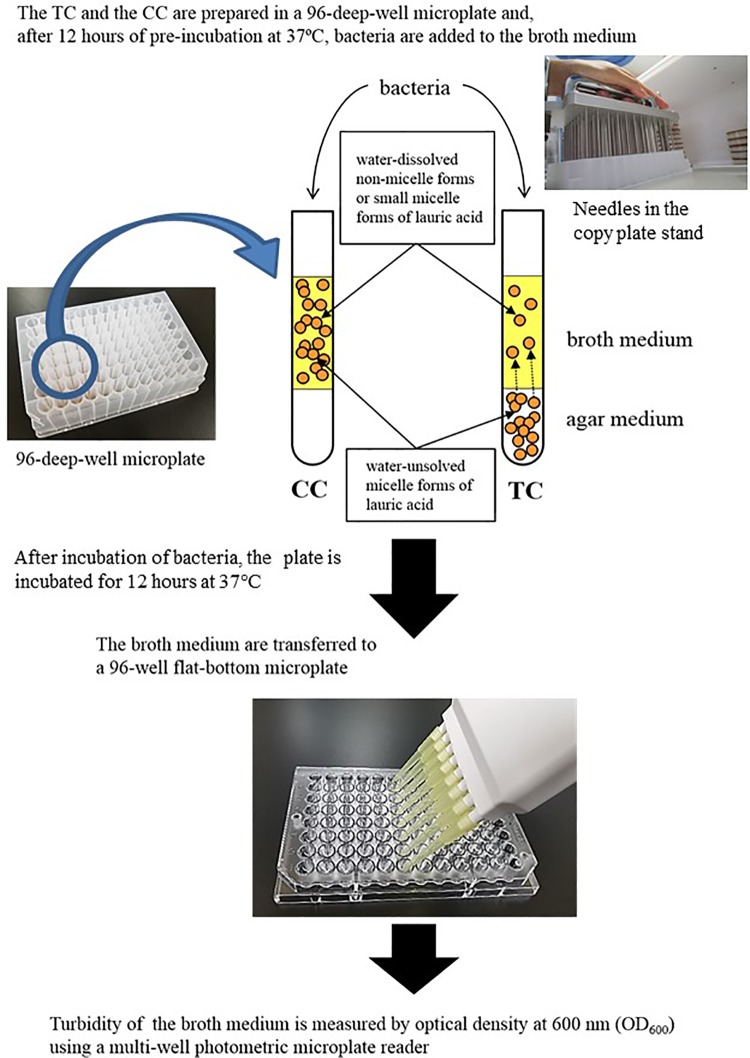
Figure 1.
Production of the test complex (TC) and control complex (CC), and measurements of turbidity and amount of FA in the broth portion of the complexes. Two types of test media were developed and added to the wells of 96-well deep-well plates. For turbidity in the top layer of both complexes, the complexes were incubated at 37°C. Then, at the indicated time, the top layer portions of both complexes were harvested to measure the turbidity at OD600 using a multi-well photometric microplate reader.
Materials and Methods
Bacteria, Media, and Fatty Acids
The bacteria tested in this study are listed in Tables 1 and 2. Cultures of 10 human pathogenic bacteria and Streptococcus salivarius were supplemented with a stock solution of 2 × concentrated Todd’s Hewitt broth medium (BD Biosciences, Franklin Lakes, NJ, USA), 0.2% yeast extract (THYbroth medium), and glycerol, and then stored at −20°C. The stored bacteria (50 μL) were inoculated into 5 mL of THY broth medium and cultured for 14 h at 37°C for use in the experiments. The human dominant gut bacteria were obtained from the American Type Culture Collection (Manassas, VA, USA), the Japan Collection of Microorganisms (Tsukuba, Japan), and the German Collection of Microorganisms and Cell Cultures GmbH (Braunschweig, Germany). Bacterial recovery was performed in accordance with the instructions of the distributors.
Table 1.
List of 10 Human Pathogenic Bacteria and Streptococcus Salivarius that were used in this Study.
| Species | Strain |
|---|---|
| Staphylococcus aureus | ATCC6538P |
| Streptococcus agalactiae | A909 |
| Streptococcus mutans | MT8148 |
| Streptococcus pneumoniae | TIGR4 |
| Streptococcus pyogenes | NIH35 |
| Streptococcus salivarius | HHT |
| Streptococcus sanguinis | ATCC10558 |
| Escherichia coli | ATCC35218 |
| Klebsiella oxytoca | K7 |
| Klebsiella pneumoniae | ATCC4352 |
| Serratia marcescens | ATCC8100 |
Table 2.
List of Human Gut Bacterial Species and Strains.
| Species | Strain | |
|---|---|---|
| Bacteroides caccae | JCM9498 | |
| Most dominant species in human gut microbes |
Bacteroides dorei | JCM13471 |
| Bacteroides finegoldii | JCM13345 | |
| Bacteroides fragilis | JCM11019 | |
| Bacteroides intestinalis | JCM13265 | |
| Bacteroides ovatus | JCM5824 | |
| Bacteroides stercoris | JCM9496 | |
| Bacteroides thetaiotaomicron | JCM5827 | |
| Bacteroides uniformis | JCM5828 | |
| Bacteroides vulgatus | JCM5826 | |
| Bacteroides xylanisolvens | JCM15633 | |
| Blautia hansenii | JCM14655 | |
| Clostridium asparagiforme | DSM15981 | |
| Clostridium nexile | ATCC27757 | |
| Collinsella aerofaciens | JCM7790 | |
| Coprococcus comes | ATCC27758 | |
| Dorea formicigenerans | ATCC27755 | |
| Dorea longicatena | DSM13814 | |
| Eubacterium cylindroides | JCM10261 | |
| Eubacterium siraeum | ATCC29066 | |
| Parabacteroides distasonis | JCM5825 | |
| Parabacteroides johnsonii | JCM13406 | |
| Parabacteroides merdae | JCM9497 | |
| Roseburia intestinalis | DSM14610 | |
| Ruminococcus gnavus | ATCC29149 | |
| Ruminococcus lactaris | ATCC29176 | |
| Ruminococcus productus | JCM1471 | |
| Ruminococcus torques | ATCC27756 | |
| Lactic acid bacteria | Enterococcus faecalis | ATCC700802 |
| Lactobacillus casei subsp. casei | JCM1134 | |
| Lactobacillus casei subsp. rhamnosus | ATCC7469 | |
| Lactobacillus gasseri | JCM1130 | |
| Lactobacillus johnsonii | JCM8794 | |
| Lactobacillus plantarum | JCM1158 | |
| Lactobacillus reuteri | JCM1149 | |
| Lactococcus lactis | JCM1112 | |
| Leuconostoc mesenteroides subsp. mesenteroides | JCM6124 | |
| Bifidobacteria | Bifidobacterium adolescentis | JCM1275 |
| Bifidobacterium animalis subsp. lactis | JCM10602 | |
| Bifidobacterium bifidum | JCM1254 | |
| Bifidobacterium breve | JCM1192 | |
| Bifidobacterium catenulatum | JCM1194 | |
| Bifidobacterium infantis | ATCC15697 | |
| Bifidobacterium longum | JCM1217 | |
| Bifidobacterium pseudocatenulatum | JCM1200 | |
| Bifidobacterium pseudolongum | JCM1205 | |
| Butyrate-producing bacteria |
Clostridium bolteae | JCM12243 |
| Clostridium indolis | JCM1380 | |
| Clostridium ramosum | JCM1298 | |
| Pathogen |
Clostridium difficile | JCM1296 |
| Clostridium perfringens | JCM1290 | |
| Fusobacterium nucleatum subsp. nucleatum | JCM8532 |
Human gut microbes (Table 2), including the most dominant species in the human intestinal indigenous microbiota17 and bifidobacteria, were pre-cultured as described previously18 in Gifu anaerobic medium (GAM) at 37°C under anaerobic conditions, because the microbes cannot be grown by THY. For elimination of dissolved oxygen from GAM, GAM bouillon (#05422; Nissui Pharmaceutical, Tokyo, Japan) was completely dissolved in deionized water and sterilized by autoclaving at 115°C for 15 min. When the temperature of the autoclave dropped to 97°C, the GAM broth medium was immediately transferred into an anaerobic chamber (INVIVO2 400; The Baker Company, Sanford, ME, USA) and incubated overnight at 37°C under anaerobic conditions (0% O2, 5% H2, 10% CO2, and 85% N2) to eliminate any dissolved oxygen.
LA and CA were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). LA has antimicrobial activity for several Staphylococcus including Staphylococcus aureus and Streptococcus bacteria, whereas CA has antimicrobial activity for Streptococcus pyogenes and Streptococcus sanguinis, but not Staphylococcus aureus, Streptococcus agalactiae, Streptococcus mutans, or Streptococcus salivarius 1–5. Since LA, but not CA, inhibits growth of S. aureus, we selected the two compounds to show the differentiation of antimicrobial activity for S. aureus as shown in the following experiment. The FAs were dissolved in 100% ethanol to final concentrations of 2.5, 0.75, and 0.25 M. The ethanol–FA solutions were concentrated 1000-fold with culture media to 2.5, 0.75, and 0.25 mM, respectively, for use in the antimicrobial experiments. We confirmed that the bacteria were able to grow in media containing 0.1% ethanol to the same extent as with the media alone (data not shown).
Production of the Test Complex and Control Complex, and Measurements of Turbidity and FA Content in the Broth Portion of Both Complexes
Two types of complexes were added to the wells of 96-deep-well plates (Fig. 1). The bottom layer of the Test Complex (TC) consisted of 100 μL of medium (either THY or GAM) supplemented with 2% agar and various concentrations of LA or CA, and the top layer consisted of 300 μL of broth medium. The bottom layer of the Control Complex (CC) consisted of 100 μL of agar medium (either THY or GAM) in the bottom layer, and the top layer consisted of 300 μL of broth medium with various concentration of LA and CA. To prepare the agar medium of both complexes, the agar medium was autoclaved (THY at 121°C for 15 min; GAM at 115°C for 15 min). The agar medium was autoclaved and allowed to cool to 50°C. Then, a 1/1000 volume of 100% ethanol or 100% ethanol and 1000-fold concentrated MCFAs were added to the cooled medium, which was gently agitated. Then, 100 μL of the solution was pipetted into the wells of a 96-well deep-well microplate. Human gut microbial species as described in Table 2 can be grown by GAM medium under anaerobic condition. For the GAM medium, the above procedure was performed under anaerobic conditions as described previously.
For measurement of the turbidity of the broth medium in the top layer of both complexes, the complexes were incubated at 37°C. Then, at the indicated times, the top layer portions of the complexes were harvested to measure the turbidity at the optical density of 600 nm (OD600) using a multi-well photometric microplate reader (Multiskan GO; Thermo Fisher Scientific, Waltham, MA, USA). To measure the amount of FAs in the THY broth medium and GAM broth medium portion of the TC, the complex was incubated at 37°C for the indicated time (10 min, 2, 6, 8, 12, and 24 h) and the THY broth medium and GAM broth medium portion was harvested. Then, the amount of FAs in the medium was measured using the Free Fatty Acid Assay Kit (STA-618; Cell Biolabs, Inc., San Diego, CA, USA), in accordance with the manufacturer’s instructions.
Inoculation of S. aureus in the Complexes and Bacterial Colony Counting
S. aureus is very common human pathogenic bacteria, and widely used as a test organism in antimicrobial screening methods. The stored S. aureus was grown for 14 h in THY broth medium. Then, the culture medium containing the bacteria was inoculated into the 300 μL top layer portion of the TC and CC, as the starting concentration of bacteria in the top layer portion is fixed to 0.05–0.06 of OD600, which is 1–3 × 105 CFU/mL. The complexes were produced with various concentrations of LA and CA and pre-incubated at 37°C for 12 h before bacterial inoculation. After inoculation of 0.3–1 × 105 CFU bacteria into the 300 μL top layer portion (1–3 × 105 CFU/mL), the complexes were incubated at 37°C for 12 h. Afterward, the bacterial suspensions in the broth portion of the complexes were serially diluted by 10-fold and plated on the THY agar plates. After incubation at 37°C for 24 h, the number of bacterial colonies was counted.
Bacterial Test and Turbidity Measurements
The turbidity of the bacterial cultures was measured as shown in Fig. 1. The bacteria presented in Table 1 were grown for 14 h in THY broth medium. The bacterial culture was inoculated into 300 μL of the top layer of the TC containing various concentrations of MCFAs in the bottom layer. The concentrations of the indicated bacteria are fixed at 0.05–0.06 of OD600, and the number of colonies depends on the bacterial species: S. aureus, S. agalactiae, Streptococcus pneumoniae, S. pyogenes, Klebsiella pneumoniae, and Klebsiella oxytoca, 0.3–1 × 105 CFU/300 μL of the top layer; S. salivarius, S. sanguinis, Escherichia coli, and Serratia marcescens, 0.3–1 × 107 CFU/300 μL of the top layer. Three wells were inoculated with bacteria but did not contain MCFAs in the bottom layer, serving as a bacterial growth controls, and the three wells were not inoculated with any bacteria but did not contain MCFAs in the bottom layer portion, serving as a blank. The plate was then incubated at 37°C and after 12 h the bacterial culture was harvested from the top layer and transferred to a microtiter plate for turbidity measurement. The turbidity was measured at OD600 using a microplate reader (Multiskan GO microplate reader, Thermo Fisher Scientific). The amount of bacteria in the broth medium of the top layer in the TC was assessed using the colony counting method and a correlation diagram was used to relate the number of bacterial colonies to the turbidity. The bacterial culture in the top layer was harvested and the turbidity was measured at OD600. The number of bacteria in the top layer portion of the TC was measured by the colony counting method and the turbidity in the top layer portion without MCFAs in the lower layer was measured with a microplate reader. With the use of a correlation diagram, the number of bacterial colonies in the culture broth was calculated from the turbidity of the bacterial culture in the top layer of the TC with the MCFAs in the bottom layer.
For measuring the turbidity of cultures of human gut microbes, the TC contained 100 μL of oxygen-free GAM agar medium(bottom layer portion) and 300 μL of oxygen-free GAM broth medium (top layer portion) in the wells of 96-deep-well plates. The pre-cultures of bacteria at the starting point (0.05–0.06 of OD600) indicated in Table 2 were inoculated in the GAM broth medium using a copy plate stand (Tokken, Chiba, Japan) and cultured under anaerobic conditions for 24 h (Fig. 1). Bacterial growth was estimated by measurement of turbidity at OD600 using a Multiskan GO microplate reader (Thermo Fisher Scientific). The plateau of bacterial growth in the absence of LA greatly differed among bacterial species (data not shown). Therefore, the relative growth was calculated, which indicates bacterial growth in the presence of LA, as compared with no LA.
Statistical Evaluations
All data are expressed as mean ± standard error of the mean (SEM). All statistical analyses were conducted using IBM SPSS Statistics for Windows, version 24.0 (IBM Corporation, Armonk, NY, USA). Statistical comparisons among the groups were performed with the Student’s t-test and multiple intergroup comparisons were made using one-way analysis of variance followed by Tukey’s multiple comparison test. A probability (p) value of <0.05 was considered statistically significant.
Results
Comparison of Turbidity by Adding MCFAs between the TC and CC
Turbidity of broth medium in TC and CC after adding MCFAs was measured. Furthermore, the turbidity of the TC was compared with that of the CC in which MCFAs were suspended in the broth medium portion for 12 h.
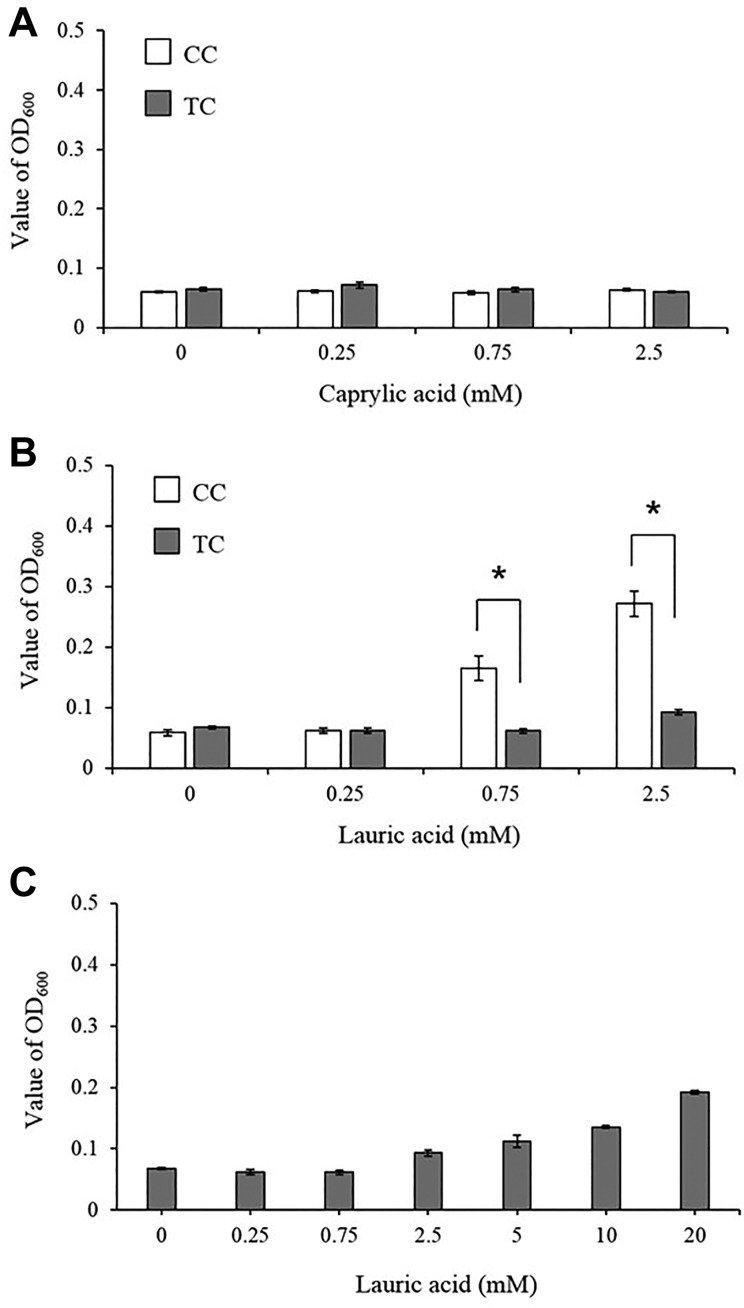
The turbidity of the comparatively water-soluble CA when suspended in the top layer portion was not changed by increasing the concentration of CA (Fig. 2A). Meanwhile, the turbidity of LA, which is poorly water soluble, had increased to more than 0.17 at 0.75 mM or more in the top layer portion of the CC. However, the turbidity of the top layer in the TC did not increase as much as the concentration of LA (0.75 mM and equivalent to 2.5 mM) (Fig. 2B).
Figure 2.
Turbidity of the broth medium portion of the TC and CC complexes after incubation with MCFAs. In the TC, MCFAs were encapsulated in the THY agar medium, while in the CC, MCFAs were suspended in the THY broth medium. Both the TC and CC complexes were then incubated at 37°C for 12 h. Then, the turbidity of the broth medium portion in the TC and in the CC complexes were measured and compared. The turbidity of CA and LA in the broth medium of the two complexes is shown in (A) and (B), respectively. (C) shows the turbidity of TC with increasing concentrations of LA. Data are presented as mean ± SEM of six samples (two independent experiments performed in triplicate). The Student’s t-test was used to evaluate differences between groups. *p < 0.05.
The change in turbidity of the top layer portion was measured when a higher concentration of LA was added to the bottom layer portion of the TC. As shown in Fig. 2C, the turbidity of the top layer portion in the TC increased with the concentration of LA. However, the turbidity of 20 mM LA in the TC was still lower than that at 2.5 mM in the CC.
Amount of MCFAs in the Top Layer Portion of the TC
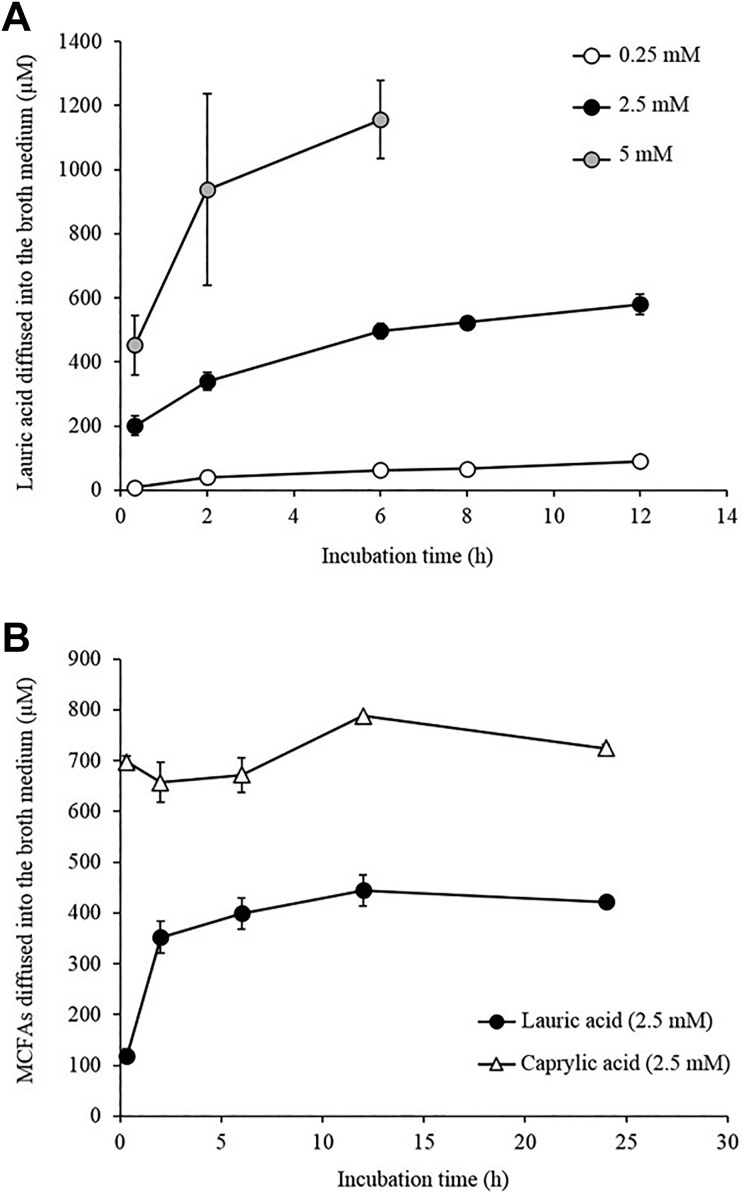
Next, after adding LA to the TC, the amount of LA in the top layer portion was examined. LA concentrations of 0.25, 2.5, and 5 mM were added to the bottom layer portion of the TC, which was then incubated at 37°C. Then, at 2, 4, 6, 8, and 12 h, the top layer portion was harvested and the amount of LA was measured. As shown in Fig. 3A, the amount of LA migrating to the top layer portion of the TC gradually increased with time. When 5 mM of LA was added, the amount of LA in the top layer portion was 1.1 mM at 6 h after the addition. Furthermore, the amount of LA in the top layer portion increased to nearly 44 μM when added at 0.25 mM and to 440 μM when added at 2.5 mM, respectively, at 12 h after the addition. However, after 12 h, there was no increase in the amount of LA in the top layer portion, which remained almost constant until after 24 h (Fig. 3B). The results showed that the amount of LA diffusing from the bottom layer portion to the top layer portion was almost 17–18% of the total. The amount of LA in the top layer portion was also determined after adding LA to the CC, and the concentration in the top layer portion at 2, 6, 8, 12, and 24 h after the addition was the same as that when initially added (data not shown).
Figure 3.
MCFA content in the top layer portion. (A) LA equivalent to concentrations of 0.25, 2.5, and 5 mM was added to the bottom layer portion of the TC and incubated at 37°C. Then, at 10 min, 2, 6, 8, and 12 h, the top layer portion was harvested and the amount of LA in the THY broth medium was measured. (B) LA and CA equivalents to concentrations of 2.5 mM were added to the bottom layer portion of the TC and incubated at 37°C. Then at 10 min, 2, 6, 12, and 24 h, the top layer portion was harvested and the amount of the FAs in the medium was measured. Data are presented as mean ± SEM of six samples (two independent experiments performed in triplicate).
With the comparatively water-soluble CA, 0.65 mM was present in the top layer portion at 2 h after the addition of 2.5 mM, and there was hardly any change in the concentration until after 24 h (Fig. 3B).
Comparison of the Antibacterial Effects of MCFAs between the TC and CC
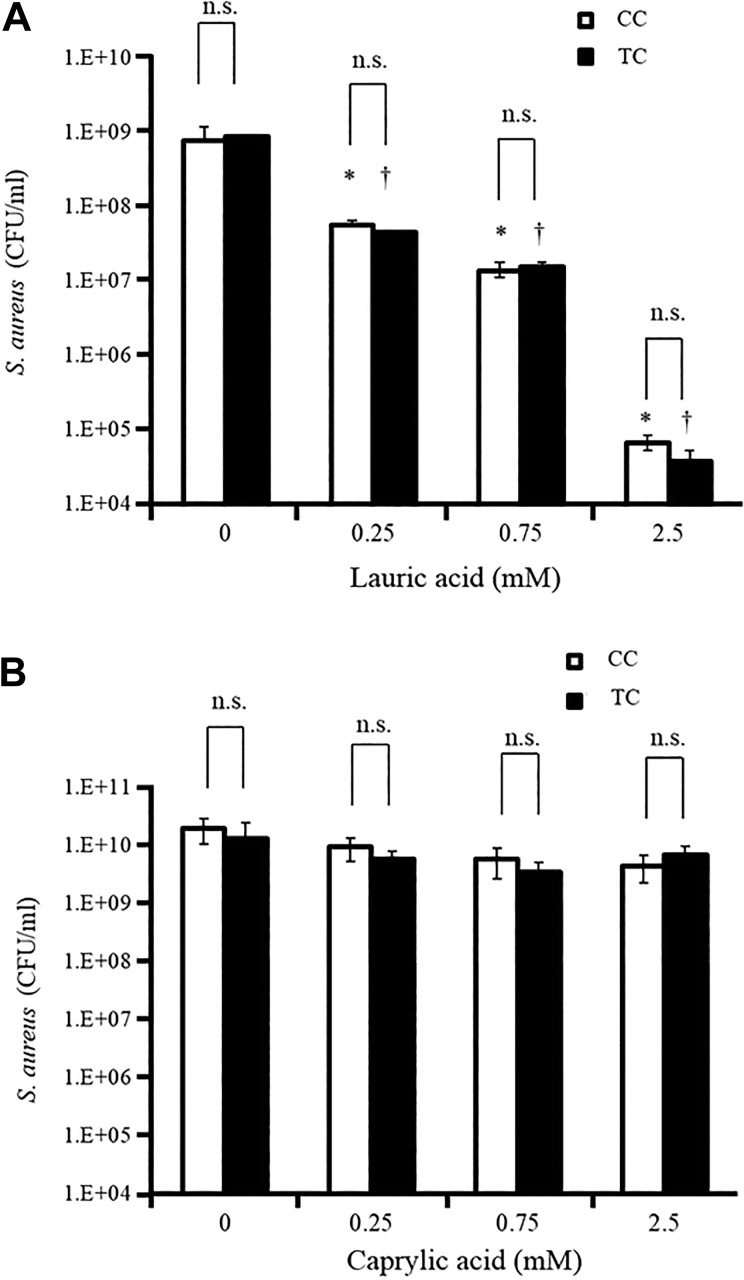
Since it is well known that LA has antimicrobial effects against S. aureus 4,16,19,20, the activity of LA against S. aureus between the TC and CC was compared. LA was added at concentrations of 0.25, 0.75, and 2.5 mM to the TC and CC, which were then incubated at 37°C for 12 h. Thereafter, the complex was mixed gently and S. aureus (0.3-1 × 105 CFU) was added to the top layer portion. After incubation at 37°C for 12 h, the number of bacterial colonies in the top layer portion was counted by the colony counting method. Surprisingly, the broth medium with LA diffused from the bottom layer portion in TC and broth medium with LA in CC inhibited growth of S. aureus equally. (Fig. 4A). In addition, CA, which has no antimicrobial effect against S. aureus, did not suppress the growth of S. aureus in either complex (Fig. 4B).
Figure 4.
Comparison of antibacterial effects of MCFAs between the TC and CC. LA (A) and CA (B) were added at 0.25, 0.75, and 2.5 mM in both complexes, and incubated at 37°C for 12 h. Thereafter, the complex was mixed gently and S. aureus (0.3–1 × 105 CFU) was added to the top layer portion. After incubation at 37°C for 12 h, the number of bacterial colonies in the top layer portion was counted with the colony counting method. Data are presented as mean ± SEM of six samples (two independent experiments performed in triplicate). *p < 0.05 compared with the white-colored bar data at LA 0; † p < 0.05 compared with the black-colored bar data at LA 0; n.s. = not significant.
Antimicrobial Activity of LA Determined by Absorbance Measurement
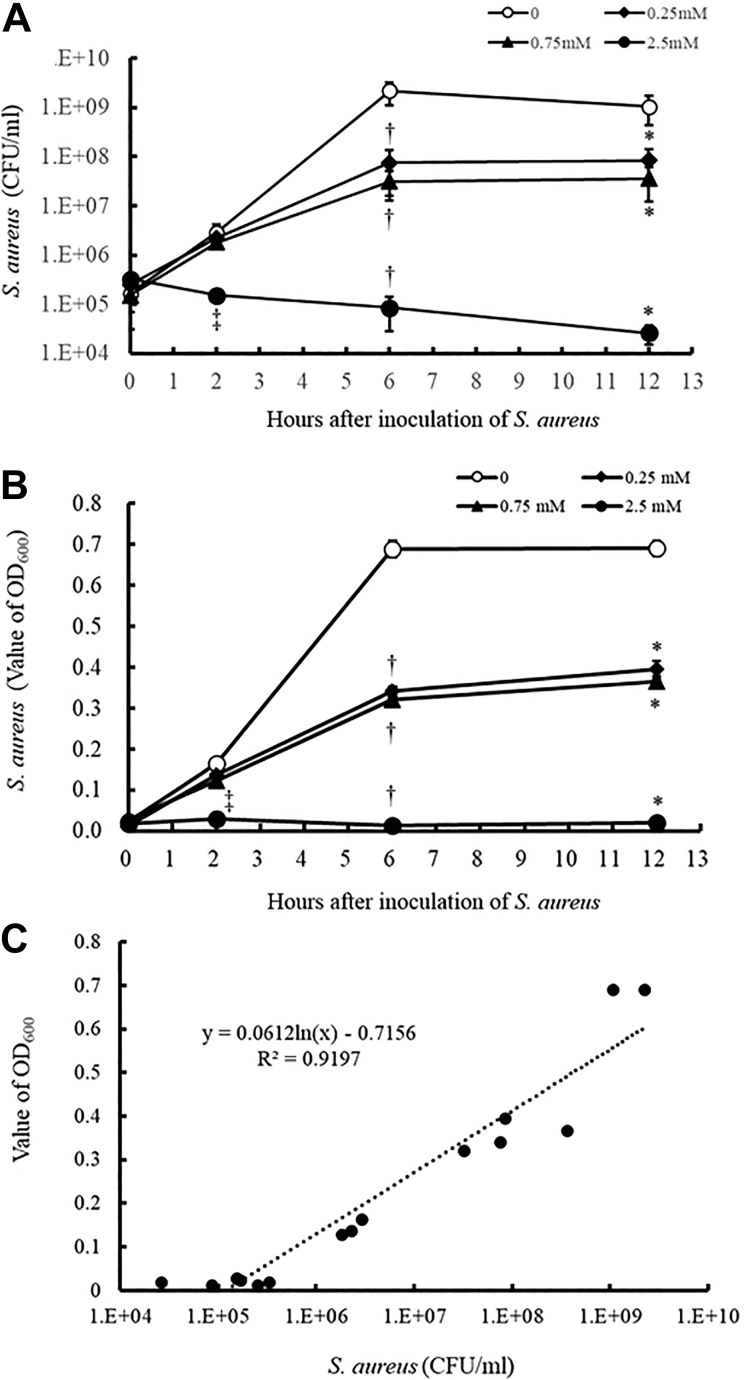
The method we developed allows us to assess the antimicrobial activity of the water-soluble part of MCFAs based on an absorbance measurement. The TC containing LA was added to the wells of 96-deep-well microplates. After incubation at 37°C for 12 h, S. aureus was inoculated into the top layer portion of the TC. At 0, 2, 6, and 12 h of incubation, the top layer portion containing the bacteria was harvested and used to count the number of S. aureus by a colony count method and to measure absorbance at OD600. As shown in Figs. 5A and B, the number of bacteria and the OD600 decrease with increasing amounts of LA added to the agar. Furthermore, the OD600 values at the indicated concentrations of LA were in proportion to the number of bacteria (Fig. 5C). The same results have been shown in the case of S. sanguinis and E. coli (Supplemental Fig. 1), suggesting that the absorbance measurements can provide quite an accurate estimate of the number of bacterial cells.
Figure 5.
Antimicrobial activity of LA assessed by colony counting methods and absorbance measurement. The TC containing LA was placed in the wells of 96-well deep-well microplates. After incubation for 12 h at 37°C, S. aureus was inoculated into the top layer portion of the TC. After incubation for 0, 2, 6, and 12 h, the top layer portion containing the bacteria was harvested and the number of S. aureus colonies was counted by the colony count method (A) and absorbance at OD600 was measured (B). Panel C shows the correlation between the numbers of bacteria by the colony count method and the OD600 values from the data of panels A and B. Data in (A) and (B) are presented as mean ± SEM of six samples (two independent experiments performed in triplicate). *p < 0.05 compared with the data of LA 0 at 12 h; † p < 0.05 compared with the data of LA 0 at 6 h; ‡ p < 0.05 compared with the data of LA 0 at 2 h.
Antimicrobial Activity of LA against Human Pathogenic Bacteria and Oral Streptococci
Numerous studies have reported the antibacterial effects of LA13,15,16,21,22 and some have demonstrated that LA has antimicrobial effects against Gram-positive streptococci, but not many Gram-negative bacilli (i.e., E. coli, K. oxytoca, K. pneumoniae, and S. marcescens). Therefore, we investigated whether the antimicrobial effects against these bacteria can be accurately assessed with our novel measurement method.
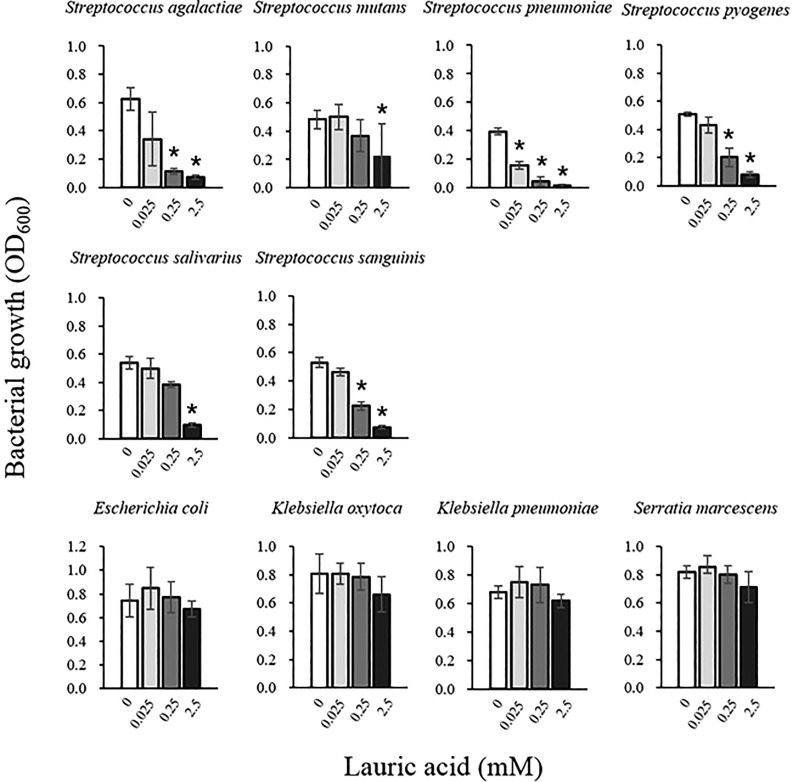
Nine human pathogenic bacteria and S. salivarius were seeded in the TC containing various concentrations of LA, and the turbidity change of the broth medium portion after incubation at 37°C for 12 h was measured. The concentrations of the indicated bacteria at the starting point (0.05–0.06 of OD600) is the following: S. aureus, S. agalactiae, S. pneumoniae, S. pyogenes, K. pneumoniae, and K. oxytoca, 1–3 × 105 CFU/mL; S. salivarius, S. sanguinis, E. coli, and S. marcescens, 1–3 × 107 CFU/mL. As shown in Fig. 6, the proliferation of S. agalactiae, S. mutans, S. pneumoniae, S. pyogenes, S. salivarius, and S. sanguinis was significantly inhibited by the addition of 0.25 mM and 2.5 mM LA (Fig. 6). On the other hand, the growth of E. coli, K. oxytoca, K. pneumoniae, and S. marcescens was not significantly suppressed even with the addition of 2.5 mM LA (Fig. 6). We had confirmed that LA affects the growth of S. aureus, S. mutans, S. pneumoniae, S. pyogenes, S. salivarius, and S. sanguinis, but not that of E. coli, K. pneumoniae, K. oxytoca, and S. marcescens using a disk diffusion antibacterial test16. These results suggest that our method can correctly evaluate the antimicrobial effects of LA.
Figure 6.
Antimicrobial activity of LA against human pathogenic bacteria and oral streptococci. LA equivalent to concentrations of 0.25, 2.5, 5 mM was added to the bottom layer portion of the TC in the wells of 96-well deep-well microplates. After incubation for 12 h at 37°C, nine human pathogenic bacteria and S. salivarius were inoculated into the top layer portion of the TC. After incubation for 12 h, the top layer portion containing the bacteria was harvested and the turbidity was measured at OD600. Data are presented as mean ± SEM of six samples (two independent experiments performed in triplicate). *p < 0.05 compared with the data of LA 0.
Antimicrobial Effects of LA on Human Gut Microbes
Measurement of the antimicrobial activity of LA by the colony count method or diffusion disk test is time-consuming and laborious; thus, it is extremely difficult to assess the concentrations of many types of bacteria simultaneously. Since the proposed measuring method can easily assess the antimicrobial activities of LA against a large amount of bacteria in a short time, we examined the antimicrobial activities of LA against human gut microbes.
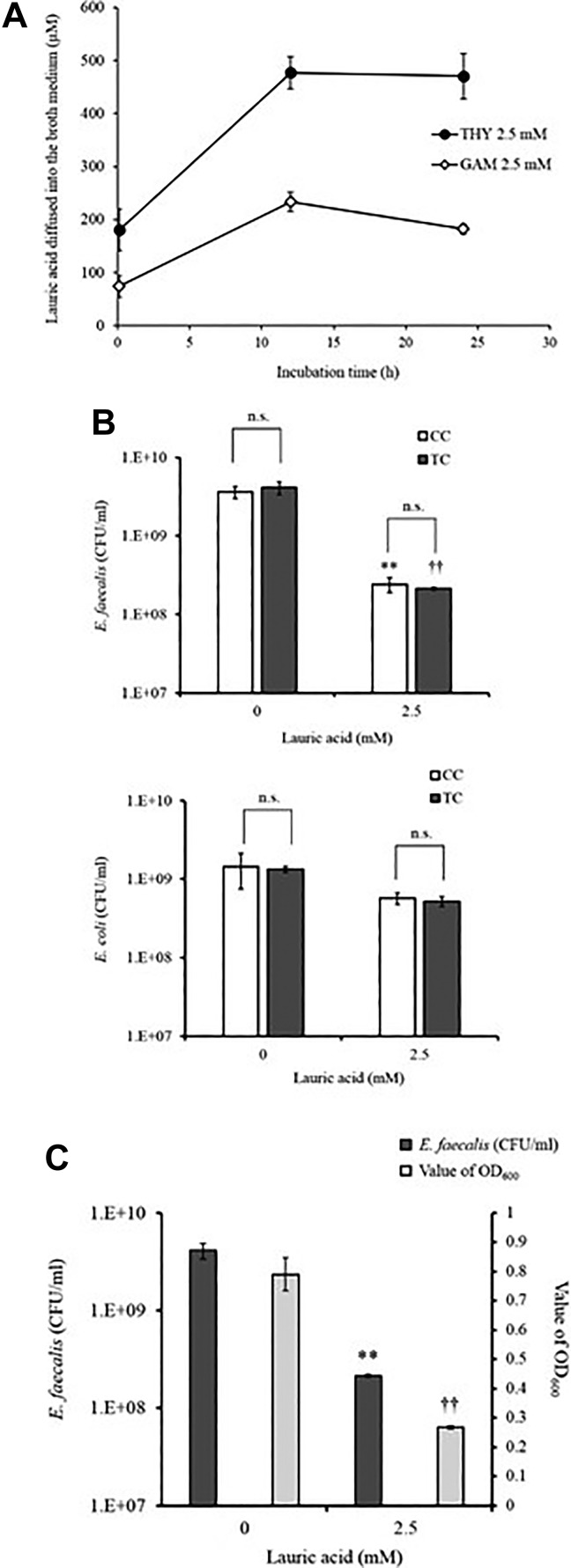
First, we investigated changes in penetration into the top layer of LA when the medium in TC was changed from THY to GAM. In Fig. 7A, LA in the bottom layer in GAM penetrated into the top layer and in THY; however, the diffused amount of LA in GAM was nearly 50% of that in THY. In order to examine how the decrease in the amount of LA in the top layer affects the antibacterial effect in TC, we created TC and CC in GAM and compared the antibacterial effect in both colony counts. Fig. 7B shows that the antimicrobial effects of Enterococcus faecalis in the broth medium of the LA-containing top layer diffused from the bottom layer in the TC and broth medium in the top layer; the antibacterial effects of the liquid medium due to the addition of LA in the CC that was equally inhibited were also evaluated. Moreover, LA did not suppress the growth of E. coli, which is resistant to LA, in either complex (Fig. 7B). We also showed that the antimicrobial effect obtained with the proposed method is comparable to that observed when LA is suspended in the top layer with the bacteria (Fig. 7C). The results suggest that it is possible to detect the antimicrobial activity in our system by GAM.
Figure 7.
LA content in the top layer and antimicrobial activity in GAM. (A) LA with 2.5 mM concentrations was added to the bottom layer of the TC and incubated at 37°C. Then, at 10 min, 12 h, and 24 h, the upper portion was harvested, and the amount of LA in the medium was measured. Closed circles are for THY 2.5 mM, and open circles are for GAM 2.5 mM. (B) LA was added at 0 and 2.5 mM in both complexes and incubated at 37°C for 12 h. Thereafter, the complex was gently mixed, and E. faecalis and E. coli (0.3–1×107 CFU) were added to the top layer. After incubation at 37°C for 12 h, the number of bacterial colonies in the top layer was counted using the colony counting method. Panel C shows the correlation between the numbers of E. faecalis by the colony count method and the OD600 values in TC. Data are presented as mean ± SEM of six samples (two independent experiments performed in triplicate). **p < 0.05 compared with the white-colored bar data at LA 0; †† p < 0.05 compared with the black-colored bar data at LA 0; n.s., not significant.
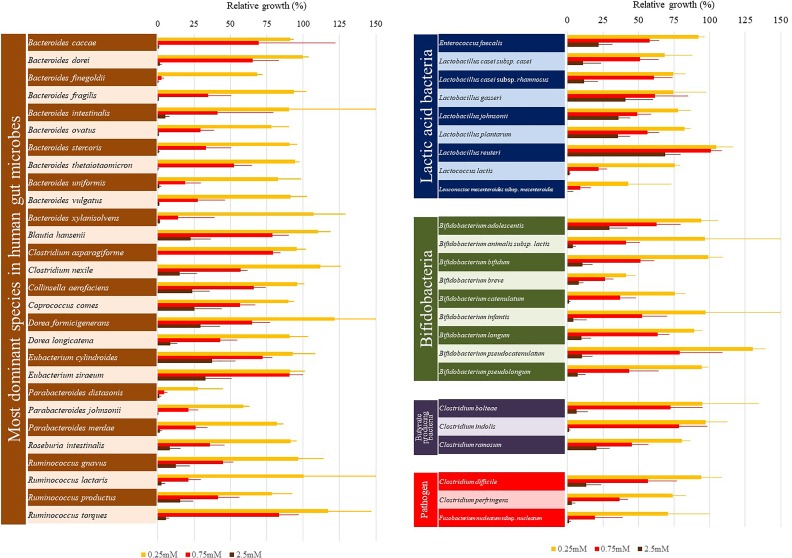
The plateau of bacterial growth in the absence of LA greatly differed among bacterial species (data not shown). Therefore, we calculated the relative growth of the bacteria in the presence of LA, as compared with that without LA (Fig. 8).
Figure 8.
Antimicrobial effect of LA on human gut microbes. LA equivalent to concentrations of 0.25, 2.5, 5 mM was added to the bottom layer portion of the TC in the wells of 96-well deep-well microplates. After incubation for 12 h at 37°C, human gut microbes were inoculated into the top layer portion of the TC to fix 0.05–0.06 of OD600. After incubation for 12 h, the top layer portion containing the bacteria was harvested and the turbidity was measured at OD600. LA was not added to some wells; these were used as controls for each bacterial growth. The relative bacterial growth in the presence vs. absence of LA was calculated. Data are presented as the mean ± SEM of six samples (two independent experiments performed in triplicate).
The growth of all of the gut microbes tested was suppressed by LA, although there were variations in the extent of suppression. The growth of gut microbes belonging to the genera Bacteroides and Parabacteroides was mostly suppressed by the addition of 2.5 mM LA to the TC (Fig. 8). In contrast, the growth of lactic acid bacteria (genus Lactobacillus) in the presence of 2.5 mM LA in the TC was in the range of 15% (Lactobacillus casei subsp. casei) to 60% (Lactobacillus reuteri), as compared with that without the addition of LA (Fig. 8). The growth of E. faecalis in the presence of 2.5 mM LA in the TC was reduced by 75%, while the growth of Lactococcus lactis was completely inhibited. In both of the Eubacterium species tested, the growth in the presence of 2.5 mM LA in the TC was reduced by about 70%, as compared with that without the addition of LA (Fig. 8). The growth of Bifidobacterium adolescentis, Blautia hansenii, Clostridium ramosum, Collinsella aerofaciens, Coprococcus comes, and Dorea longicatena was reduced by about 75% by the addition of 2.5 mM LA to the TC. Meanwhile, the growth of Clostridium perfringens and Fusobacterium nucleatum subsp. nucleatum was completely inhibited by the addition of 2.5 mM LA to the TC, and the growth of Clostridium difficile was reduced by about 80% of that without LA (Fig. 8).
Discussion
In the present study, a novel method is described for the measurement of the antimicrobial activity of LA with the use of a microplate reader. With this assay system, the antimicrobial activity of LA diffusing from the bottom layer portion in TC was comparable to that of LA dissolved in the top layer portion in CC. Importantly, we showed that the amount of bacteria in the top layer portion of the TC was corrected with turbidity.
The disc diffusion antimicrobial test has traditionally been used to evaluate the antibacterial activity of MCFAs16. This measurement method utilizes the phenomenon that penetration of LA in a disk to agar medium plate which contains 1.5–2% agar inhibits bacterial growth on the plate13–16. We confirmed the formation of a clear zone around the disk infiltrated with LA on S. aureus seeded in agar medium. It was further confirmed that the agar medium in the inhibition zone was not cloudy. Based on these results, a novel measuring method using TC was devised (Fig. 1). In the present study, LA in the bottom layer portion of the TC penetrated the top layer portion and suppressed bacterial growth in the top layer portion (Fig. 4). However, the LA contained in the agar did not cloud the top layer portion (Fig. 2). These results suggest that the proposed novel method was sufficient to monitor changes in bacterial growth in response to LA using a spectrophotometer. In our experiments, the bottom layer portion is set at 2%. Because of its fragility, 1.5% agar was not used during the experiment. In addition, we use 100 μL of agar medium and 300 μL of broth medium because of technical requirements. We make the TC and CC in each well (500 μL capacity) of a 96-well deep-well plate. Next, we inoculate bacteria in each well using the “copy plate stand” shown in Fig. 1. In this inoculation process, the height of the bottom portion should be sufficiently low so that the needles in the copy plate stand (Fig. 1) do not stab the agar. Furthermore, the volume of the broth medium was limited to 300 μL to prevent overflow during bacterial inoculation. We performed the experiments under these conditions.
The evaluation of the antimicrobial effect with the proposed method yields similar results as the colony counting method (Fig. 4). Furthermore, regarding the antibacterial effects of LA against each tested bacterial species, the results of the proposed measuring method were comparable as previously reported13,15,16,21,22. The antimicrobial activities of MCFAs are conventionally assessed by a bacterial colony counting method, as described in the introduction section. However, the bacterial colony counting method requires a great deal of agar medium and is energy- and time-consuming. Therefore, it is rather difficult to measure and compare the antibacterial activities of various FAs against many bacterial species simultaneously. The method proposed here can be used to evaluate the antimicrobial effects of FA against many bacterial species simultaneously, thanks to the 96-well plate setting. In addition, since the OD600 value correlates with the number of colonies, the antimicrobial activity can be assessed easily and accurately by measuring absorbance in a microplate reader.
Since the number of bacteria in the broth medium is correlated with the turbidity of the media, a simple measurement of turbidity with a microplate reader is generally used to calculate the number of bacteria. However, the presence of FAs muddies the broth medium, making it impossible to precisely calculate the number of bacteria. In contrast, one might believe that we have good control when we only add FA and then validate by plating out the bacteria from the other wells; thus, we can somehow correct the “muddiness” created by the FA. However, we do not agree with this opinion. We showed that adding 0.75 mM and 2.5 mM of LA to the broth medium increased the turbidity at 0.2 and 0.3 of OD600, respectively (Fig. 2), and turbidity indicates that the numbers of S. aureus are usually 3×106 CFU/mL and 107 CFU/mL, respectively (Fig. 5). This means that bacterial growth cannot be recognized when the number of bacteria is not increased to > 3 × 106 CFU/mL and 107 CFU/mL. Since the inoculation amount of S. aureus in the bacterial growth test is 1–3 × 105 CFU/mL (0.05–0.06 of OD600), in terms of turbidity values, the addition of LA indicates significant bacterial growth. Furthermore, the maximum value of OD600 in some bacteria, i.e., many strains of genus Streptococcus, is 0.4–0.6, which is similar to the turbidity level of the broth medium with LA. Therefore, we cannot determine the bacterial growth or exact level of inhibitory bacterial growth by LA. We believe that the presence of FAs, including LA, muddies the broth medium, making it impossible to precisely calculate the number of bacteria. Since LA is only partially soluble in water, the majority of the FA forms micelles in water. However, in the TC, the top layer did not become cloudy, most likely because only the soluble portion of the LA diffused from the bottom layer to the top layer. This resulted in 17%–18% (in the case of THY) and 8%–9% (in the case of GAM) of the total LA inoculated in the bottom layer being present in the top layer, where it consequently induced antibacterial activity. We also showed that the antimicrobial effect obtained with the proposed method is comparable to that observed when LA is suspended in the top layer with the bacteria. The carbon chain and methyl carboxylate of FAs bind and show no antimicrobial activity, whereas binding of the carbon chains of FAs to the carboxyl group conveys antibacterial activities23. In addition, Prasad et al. demonstrated that physical features in the agar–fatty acid complex are different from that in agar alone24. The agar gel in the presence of some MCFAs induces decreased surface tension activity, enhanced hydrophobicity, decreased water retention, and decreased gel strength. However, the amount of FAs chemically bound in the agar–fatty complex is very few (almost 0.1–0.2%) when adding FAs to liquefied agar to make the agar–fatty acid complex24. The results suggest that formation of the agar–fatty acid complex releases water and hydrophilic substances, including hydrophilic groups of the FAs, out of the agar due to reduced water retention; thus, the presence of the hydrophilic group of the FAs greatly affects antibacterial activity. However, it is not clear how MCFAs encapsulated in agar actually come out in liquid medium; thus, in order to further improve the accuracy of this assay, it is necessary to clarify how MCFAs encapsulated in agar come out in the liquid medium, which is a subject to be examined in the future.
In the present study, we determined the antibacterial effect in THY and GAM. Although the amount of LA penetration to the top layer of the TC in the GAM is less than that in the THY medium, the antibacterial effect in TC was similar to that in the case where LA was directly added to the GAM in CC (Fig. 7). It is still unclear why the antimicrobial activity between the two experimental systems was not different even though the penetrated amount is decreased; thus, it is necessary to clarify the reason in the future. However, the results may suggest that various media may be screened for the antibacterial effects. A previous study reported the protective effects of the oral administration of LA against C. difficile in a mouse model of inflammation22, suggesting that LA and some MCFAs found in foods may be able to escape digestion and absorption, at least partially, and reach the intestinal lumen. Furthermore, in the present study, LA was shown to inhibit the growth of the dominant bacterial species in the human gut microbe, including Bacteroides caccae and Bifidobacterium dorei (Fig. 8). It was previously reported that B. caccae degrades the colonic mucus barrier and enhances pathogen susceptibility when the host takes in food lacking dietary fiber25, and that early colonization by B. dorei may contribute to autoimmune diseases in humans through the inhibition of innate immune signaling and endotoxin tolerance26. Therefore, it is possible that the intake of LA may promote the health of the host via inhibition of bacterial growth, especially that of species belonging to the genus Bacteroides. Moreover, the oral administration of B. fragilis was shown to correct gut permeability, alter microbial composition, and ameliorate autism spectrum disorder-related defects in maternal immune-activated mice27. Therefore, it is also possible that intake of LA may adversely affect the gut–microbiome–brain connection because the growth of Bacteroides fragilis was completely inhibited in the presence of 2.5 mM LA in the present study. These are, of course, hypotheses that should be further investigated; the effects of LA in the gut may anyway be complicated by the presence of many different species, and by the fact that they act in a complex community of microbes, where the effects of many substances introduced with the diet are overlapping.
Lactic acid bacteria are probiotic bacteria contained in fermented foods such as yogurt and pickles. The results of the present study revealed that LA has low antimicrobial activity against Lactobacilli and E. faecalis (Fig. 8). Lactic acid bacteria are the predominant species in the human small intestine and inhibit enteritis through the induction of interferon β28. Therefore, LA may enhance this anti-inflammatory activity through antimicrobial activities against other bacteria that compete with lactic acid bacteria. In humans, although colonic microbiota lactic acid bacteria are non-dominant, it may be possible to increase the concentrations of prebiotic lactic acid bacteria with the selective antimicrobial activity of LA. B. adolescentis, which is relatively resistant to LA (Fig. 8), is the sixth most dominant bacterial species in the feces of healthy Japanese adults29, and this bacterium strongly induces the proliferation of intestinal Th17 cells when colonized alone in the murine intestine. The induction of Th17 cells bolsters the host mucosal defenses or may lead to the development of autoimmune diseases30. Since LA has antimicrobial activities against many other bacterial species in the colonic microbiota that compete with B. adolescentis, it is possible that LA may support the growth/colonization of B. adolescentis in the colon and therefore induce proliferation of Th17 cells. C. ramosum and C. comes produce butyric acid18,31, and are believed to contribute to the suppression of enteritis through induction of regulatory T cells in the host intestinal tract. Therefore, it is not necessarily that they would proliferate in the presence of LA, but maybe they simply would not be negatively affected by it. C. perfringens is a causative agent of food poisoning and it was previously reported that F. nucleatum subsp. nucleatum is associated with colorectal cancer in addition to periodontal disease32. Interestingly, in this study we observed that both these bacteria (C. perfringens and F. nucleatum) were completely inhibited by LA (Fig. 8). Future studies are necessary to indicate whether the suppression of these bacteria would be associated with a change in disease risk, and whether LA could play a role in this modulation.
Some MCFAs have antimicrobial activities against various pathogenic bacterial constituents of the skin and may contribute to the control of the skin bacterial flora 5,10,11,33. These molecules are also contained in some foods such as coconut oil and milk34–38. Thus, the diversity of the antibacterial effects of FAs on various bacteria might contribute to modulate the composition of the intestinal flora and promote health. There are not only LA but also many antimicrobial free fatty acids and monoglycerides39; thus, it still remains unknown whether the suppression of these bacteria would be associated with a change in disease risk, and whether LA could play a role in this modulation. Future studies are warranted to investigate the antibacterial activities of other FAs against different bacteria, both individually and in complex cultures. The future studies that we indicated above will bring important suggestions. In other words, if our method could measure the accumulated bacterial inhibition by FAs and other compounds using bacterial mixtures, it may be possible to test new hypotheses regarding LA and other compounds in gut health and change of in vivo disease risk.
Conclusion
In conclusion, the proposed method for measuring the antimicrobial effect of LA can be used to quickly and simultaneously evaluate a large number of types of FAs with very simple preparation steps as compared with more conventional methods. In addition, the antimicrobial activity of LA against human gut microbes was determined with the proposed method, which showed that LA has low antimicrobial activity against lactic acid bacteria, but not Bacteroides and Clostridium. These results suggest that LA might contribute to intestinal health, as confirmed by the proposed method.
Supplemental Material
Supplemental_Fig._1 for Measuring the Antimicrobial Activity of Lauric Acid against Various Bacteria in Human Gut Microbiota Using a New Method by Miki Matsue, Yumiko Mori, Satoshi Nagase, Yuta Sugiyama, Rika Hirano, Kazuhiro Ogai, Kohei Ogura, Shin Kurihara and Shigefumi Okamoto in Cell Transplantation
Acknowledgments
We would like to thank Ms. Yoshie Yuasa, Mr. Yuusuke Kotani, and Mr. Kazuki Hayashi for the technical assistance. We also thank Enago for the English editing.
Footnotes
Ethical Approval: All methods and experimental protocols for using bacteria were approved by Kanazawa University Microorganisms Safety Management Regulations and Ishikawa Prefectural University Microorganisms Safety Management Regulations, and all experiments were performed in the Biosafety level 2 (BSL2) rooms that were approved by the Regulations.
Statement of Human and Animal Rights: Statements of Human and Animal Rights is not applicable for this article.
Statement of Informed Consent: Statement of Informed Consent is not applicable for this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of article: This work was supported by grants from the Japan Society for the Promotion of Scieence (KAKENHI): 17H04428, 26462806, and Organization of Frontier Science and Innovation (O-FSI), Kanazawa University (Sakigake Project).
ORCID iD: Shigefumi Okamoto  https://orcid.org/0000-0001-9359-380X
https://orcid.org/0000-0001-9359-380X
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Welsh JK, Arsenakis M, Coelen RJ, May JT. Effect of antiviral lipids, heat, and freezing on the activity of viruses in human milk. J Infect Dis. 1979;140(3):322–328. [DOI] [PubMed] [Google Scholar]
- 2. Thormar H, Isaacs CE, Brown HR, Barshatzky MR, Pessolano T. Inactivation of enveloped viruses and killing of cells by fatty acids and monoglycerides. Antimicrob Agents Chemother. 1987;31(1):27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Isaacs CE, Litov RE, Thormar H. Antimicrobial activity of lipids added to human milk, infant formula, and bovine milk. J Nutr Biochem. 1995;6(7):362–366. [DOI] [PubMed] [Google Scholar]
- 4. Bergsson G, Arnfinnsson J, Steingrímsson O, Thormar H. Killing of Gram-positive cocci by fatty acids and monoglycerides. APMIS. 2001;109(10):670–678. [DOI] [PubMed] [Google Scholar]
- 5. Fischer CL, Drake DR, Dawson DV, Blanchette DR, Brogden KA, Wertz PW. Antibacterial activity of sphingoid bases and fatty acids against Gram-positive and Gram-negative bacteria. Antimicrob Agents Chemother. 2012;56(3):1157–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bergsson G, Arnfinnsson J, Karlsson SM, Steingrímsson O, Thormar H. In vitro inactivation of Chlamydia trachomatis by fatty acids and monoglycerides. Antimicrob Agents Chemother. 1998;42(9):2290–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Desbois AP, Smith VJ. Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Appl Microbiol Biotechnol. 2010;85(6):1629–1642. [DOI] [PubMed] [Google Scholar]
- 8. Cameron DJ, Tong Z, Yang Z, Kaminoh J, Kamiyah S, Chen H, Luo L, Zhang K. Essential role of Elovl4 in very long chain fatty acid synthesis, skin permeability barrier function, and neonatal survival. Int J Biol Sci. 2007;3(2):111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jungersted JM, Hellgren LI, Jemec GB, Agner T. Lipids and skin barrier function--a clinical perspective. Contact Dermatitis. 2008;58(5):255–262. [DOI] [PubMed] [Google Scholar]
- 10. Zouboulis CC. Acne and sebaceous gland function. Clin Dermatol. 2004;22(5):360–366. [DOI] [PubMed] [Google Scholar]
- 11. Smith KR, Thiboutot DM. Thematic review series: skin lipids. Sebaceous gland lipids: friend or foe? J Lipid Res. 2008;49(2):271–281. [DOI] [PubMed] [Google Scholar]
- 12. Koch AL. Turbidity measurements of bacterial cultures in some available commercial instruments. Anal Biochem. 1970;38(1):252–259. [DOI] [PubMed] [Google Scholar]
- 13. Huang CB, Alimova Y, Myers TM, Ebersole JL. Short- and medium-chain fatty acids exhibit antimicrobial activity for oral microorganisms. Arch Oral Biol. 2011;56(7):650–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tangwatcharin P, Khopaibool P. Activity of virgin coconut oil, lauric acid or monolaurin in combination with lactic acid against Staphylococcus aureus . Southeast Asian J Trop Med Public Health. 2012;43(4):969–985. [PubMed] [Google Scholar]
- 15. Karimi E, Jaafar HZ, Ghasemzadeh A, Ebrahimi M. Fatty acid composition, antioxidant and antibacterial properties of the microwave aqueous extract of three varieties of Labisia pumila Benth. Biol Res. 2015;48:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nagase S, Matsue M, Mori Y, Honda-Ogawa M, Sugitani K, Sumitomo N, Nakata M, Kawabata S, Okamoto S. Comparison of the antimicrobial spectrum and mechanisms of organic virgin coconut oil and lauric acid against bacteria. J Wellness Health Care. 2017;41(1):87–95. [Google Scholar]
- 17. Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gotoh A, Nara M, Sugiyama Y, Sakanaka M, Yachi H, Kitakata A, Nakagawa A, Minami H, Okuda S, Katoh T, Katayama T, et al. Use of Gifu Anaerobic Medium for culturing 32 dominant species of human gut microbes and its evaluation base short-chain fatty acids fermentation profiles. Biosci Biotechnol Biochem. 2017;81(10):2009–2017. [DOI] [PubMed] [Google Scholar]
- 19. Rouse MS, Rotger M, Piper KE, Steckelberg JM, Scholz M, Andrews J, Patel R. In vitro and in vivo evaluations of the activities of lauric acid monoester formulations against Staphylococcus aureus . Antimicrob Agents Chemother. 2005;49(8):3187–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sado-Kamdem SL, Vannini L, Guerzoni ME. Effect of alpha-linolenic, capric and lauric acid on the fatty acid biosynthesis in Staphylococcus aureus . Int J Food Microbiol. 2009;129(3):288–294. [DOI] [PubMed] [Google Scholar]
- 21. Kabara JJ, Swieczkowski DM, Conley AJ, Truant JP. Fatty acids and derivatives as antimicrobial agents. Antimicrob Agents Chemother. 1972;2(1):23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang HT, Chen JW, Rathod J, Jiang YZ, Tsai PJ, Hung YP, Ko WC, Paredes-Sabja D, Huang IH. Lauric acid is an inhibitor of Clostridium difficile growth in vitro and reduces inflammation in a mouse infection model. Front Microbiol. 2018;8:2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zheng CJ, Yoo JS, Lee TG, Cho HY, Kim YH, Kim WG. Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett. 2005;579(23):5157–5162. [DOI] [PubMed] [Google Scholar]
- 24. Prasad K, Trivedi K, Meena R, Siddhanta AK. Physical modification of agar: formation of agar-fatty acid complexes. Polym J. 2005;37:826–832. [Google Scholar]
- 25. Desai MS, Seekatz AM, Koropatkin NM, Kamada N, Hickey CA, Wolter M, Pudlo NA, Kitamoto S, Terrapon N, Muller A, Young VB, et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016;167(5):1339–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vatanen T, Kostic AD, d’Hennezel E, Siljander H, Franzosa EA, Yassour M, Kolde R, Vlamakis H, Arthur TD, Hämäläinen AM, Peet A, et al. ; DIABIMMUNE Study Group, Xavier RJ. Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell. 2016; 165(4):842–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T Codelli JA, Chow J, Reisman SE, Petrosino JF, Patterson PH, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155(7):1451–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kawashima T, Kosaka A, Yan H, Guo Z, Uchiyama R, Fukui R, Kaneko D, Kumagai Y, You DJ, Carreras J, Uematsu S, et al. Double-stranded RNA of intestinal commensal but not pathogenic bacteria triggers production of protective interferon-β. Immunity. 2013;38(6):1187–1197. [DOI] [PubMed] [Google Scholar]
- 29. Nishijima S, Suda W, Oshima K, Kim SW, Hirose Y, Morita H, Hattori M. The gut microbiome of healthy Japanese and its microbial and functional uniqueness. DNA Res. 2016;23(2):125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tan TG, Sefik E, Geva-Zatorsky N, Kua L, Naskar D, Teng F, Pasman L, Ortiz-Lopez A, Jupp R, Wu HJ, Kasper DL, et al. Identifying species of symbiont bacteria from the human gut that, alone, can induce intestinal Th17 cells in mice. Proc Natl Acad Sci USA. 2016;113(50):E8141–E8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, Kim S, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500(7461):232–236. [DOI] [PubMed] [Google Scholar]
- 32. Bullman S, Pedamallu CS, Sicinska E, Clancy TE, Zhang X, Cai D, Neuberg D, Huang K, Guevara F, Nelson T, Chipashvili O, et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science. 2017;358(6369):1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zouboulis CC, Baron JM, Böhm M, Kippenberger S, Kurzen H, Reichrath J, Thielitz A. Frontiers in sebaceous gland biology and pathology. Exp Dermatol. 2008;17(6):542–551. [DOI] [PubMed] [Google Scholar]
- 34. Aoyama T, Nosaka N, Kasai M. Research on the nutritional characteristics of medium-chain fatty acids. J Med Invest. 2007;54(3–4):385–388. [DOI] [PubMed] [Google Scholar]
- 35. Nagao K, Yanagita T. Medium-chain fatty acids: functional lipids for the prevention and treatment of the metabolic syndrome. Pharmacol Res. 2010;61(3):208–212. [DOI] [PubMed] [Google Scholar]
- 36. Babu AS, Veluswamy SK, Arena R, Guazzi M, Lavie CJ. Virgin coconut oil and its potential cardioprotective effects. Postgrad Med. 2014;126(7):76–83. [DOI] [PubMed] [Google Scholar]
- 37. Eyres L, Eyres MF, Chisholm A, Brown RC. Coconut oil consumption and cardiovascular risk factors in humans. Nutr Rev. 2016;74(4):267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ohlsson L. Dairy products and plasma cholesterol levels. Food Nutr Res. 2010;54:5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yoon BH, Jackman JA, Valle-González ER, Cho NJ. Antimicrobial free fatty acids and monoglycerides: biological activities, experimental testing, and therapeutic applications. Int J Mol Sci. 2018;19(4):1114 doi:10.3390/ijms19041114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental_Fig._1 for Measuring the Antimicrobial Activity of Lauric Acid against Various Bacteria in Human Gut Microbiota Using a New Method by Miki Matsue, Yumiko Mori, Satoshi Nagase, Yuta Sugiyama, Rika Hirano, Kazuhiro Ogai, Kohei Ogura, Shin Kurihara and Shigefumi Okamoto in Cell Transplantation