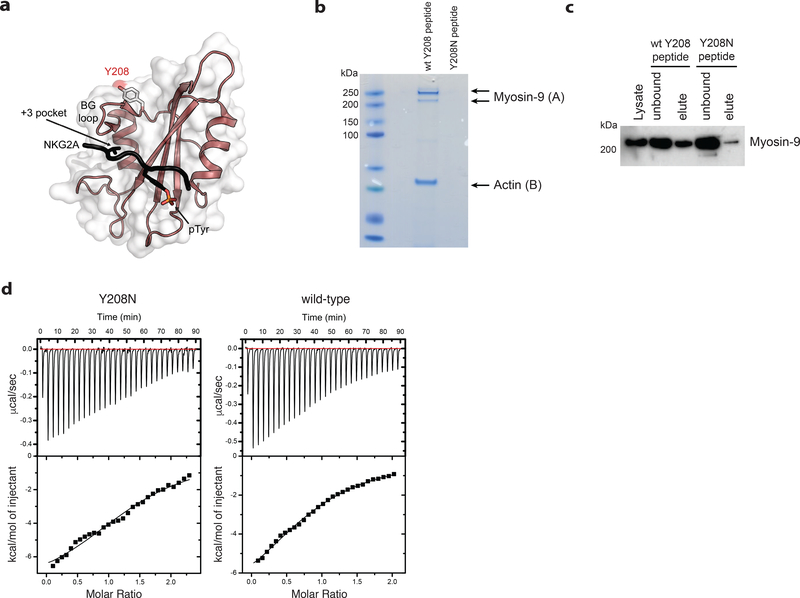
Figure 7. Ptpn6 contact with the actin-myosin cytoskeleton is mediated by Y208.
a) The structure of the Ptpn6 C-terminal SH2 domain bound to a phosphopeptide derived from the Natural Killer Group 2A (NKG2A) protein is displayed (PDB ID: 2YU7). The solvent accessible surface of the Ptpn6 SH2 domain (white) is shown overlaid on a ribbon diagram of the domain. The bound NKG2A phosphopeptide is shown in black with the phosphotyrosine residue indicated in stick representation. Tyrosine 208 is located at the C-terminal end of the BG loop. It is solvent accessible and does not contact the specificity-determining pocket (+3 pocket), nor the phosphotyrosine binding pocket. b) Coomassie staining for proteins associated with the wild-type and Y208N mutant Ptpn6 C-SH2 domains following immunoprecipitation from 108 neutrophils. Representative data from two biologically independent experiments. Peptides isolated from gel slices in (A) and (B) are described in Supplementary Table 2. c) Immunoblot for Myosin-9 in 5 × 106 neutrophils following immunoprecipitation with the biotinylated peptides corresponding to the wild-type and Y208N mutant Ptpn6 C-SH2 domain. Representative data from three biologically independent experiments. d) Isothermal titration calorimetry (ITC) analysis comparing the interaction of FcγR2b phosphopeptide with wild-type and Y208N mutant recombinant C-terminal SH2 domain. Representative data from two biologically independent experiments.