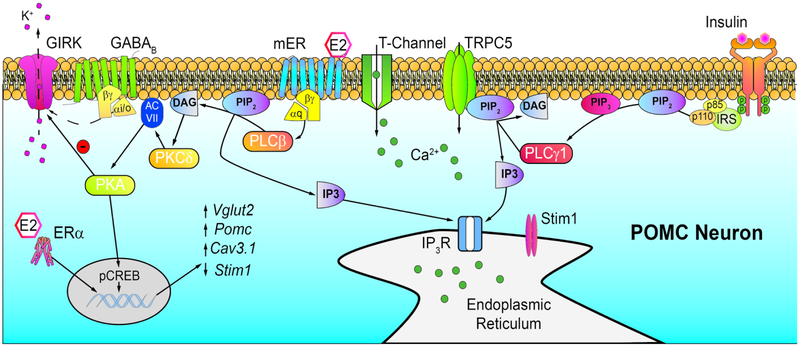
Figure 2.
E2 and insulin signaling cascades in POMC neurons. Insulin through its cognate receptor can activate phospholipase C (PLCγ) to cleave Phosphotidylinositol 4,5 biphosphate (PIP2) into Diacylglycerol (DAG) and Inositol triphosphate (IP3) opening TRPC5 channels and generating an inward cationic current to depolarize POMC neurons. Binding of E2 to Gαq-coupled mERs activates phospholipase C (PLCβ) – protein kinase C (PKCδ) – adenylyl cyclase (ACVII) – protein kinase A (PKA) signaling cascade to decouple GABAB (and μ-opioid) receptors from inhibitory G protein-coupled inwardly rectifying K+(GIRK) channels. PKA can also phosphorylate cAMP response element binding protein (pCREB) to generate new gene transcription through CRE’s. In addition, E2 binds to estrogen receptor α (ERα) in POMC neurons to increase Pomc, Vglut2, TRPC5 and CaV3.1 gene expression through ERE’s. On the other hand, stromal interacting molecule 1 (Stim1) expression is decreased, preserving TRPC5 channels as receptor operated channels for transmitting insulin’s (and leptin’s) effects. Note: E2 has similar actions in kisspeptin neurons with the exception that E2 downregulates the expression of the peptide.