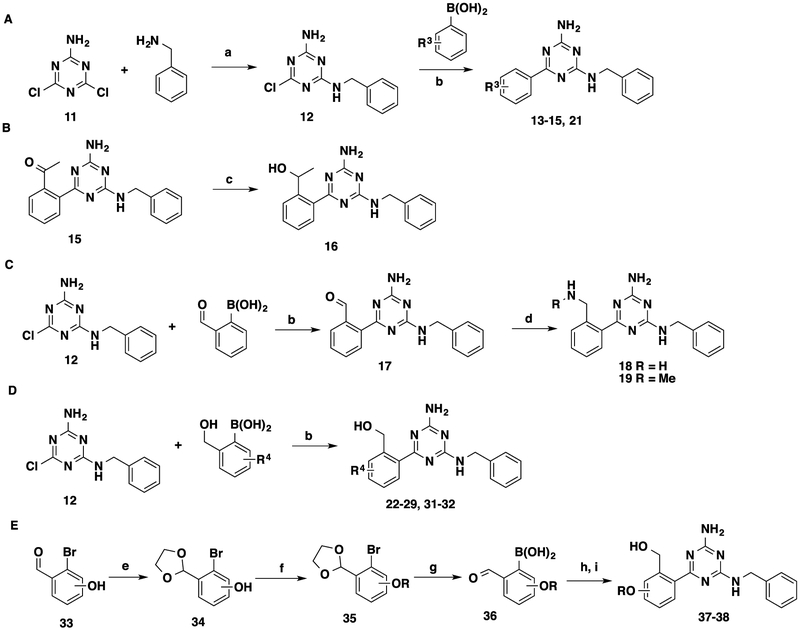
Scheme 2. Synthesis of 13–15, 21, 16, 18, 19, 22–29, 31, 32, 37, and 38a.
aReagents and conditions: (a) dioxane, reflux, 1 h, 78–94%; (b) Pd(PPh3)4, K2CO3, dioxane/H2O = 5:3, 120 °C, microwave, 20 min, 56–99%; (c) NaBH4, MeOH, 0 °C to rt, 42%; (d) NH3·H2O or NH2Me, MgSO4, NaBH4, THF, rt, 2 days, 45–67%. (e) Ethylene glycol, toluene, reflux, 4–8 h, 82–96%; (f) alkyl bromide, K2CO3, dimethylformamide, 85 °C, 34–78%; (g) i. n-BuLi, THF, −78 °C, 30 min; ii. B(OMe)3, −78 °C, 1 h, then rt, overnight; iii. HCl (3 N), rt, 1 h, 26–57%; (h) 12, Pd(PPh3)4, K2CO3, dioxane/H2O = 5:3, 120 °C, microwave, 20 min; (i) NaBH4, THF, 0 °C to rt, 23–97%.