Abstract
Background
We developed a model based on ultrasound (US) features of thyroid nodules and cervical lymph nodes to distinguish papillary thyroid carcinomas (PTC) from benign thyroid nodules.
Material/Methods
We retrospectively collected data on preoperative ultrasonographic characteristics and postoperative histological data from 1119 patients who underwent thyroidectomy in our center from January 2017 to January 2018. Variables of age, sex, and US features of thyroid nodule and lymph nodes features were analyzed. A logistic regression model was established for PTC prediction.
Results
Logistic regression analysis confirmed that age under 45 years (OR=2.22, p=0.00), hypoechogenicity (OR=3.70, p=0.00), irregular shape (OR=2.13, p=0.004), ill-defined margin (OR=2.26, p=0.08), spiculate margin (OR=3.30, p=0.00), indefinite border (OR=2.45, p=0.00), capsular invasion (OR=7.76, p=0.006), taller-than-wide shape (OR=2.94, p=0.00), solid structure (OR=2.46, p=0.001), microcalcifications (OR=3.92, p=0.00), coexistence of microcalcifications and macrocalcifications (OR=5.84, p=0.006), and central vascularity (OR=2.10, p=0.001) were independently associated with increased risks for PTC, as well as lymph nodes metastasis features (absence of an echogenic hilum [OR=3.74, p=0.027] and increased vascularization [OR=3.55, p=0.086]). The area under the curve (AUC) for the risk score diagnosis system was 0.916.
Conclusions
This predictive model is a reliable, simple, and cost-effective diagnostic tool for PTC.
MeSH Keywords: Carcinoma, Papillary; Logistic Models; Lymph Nodes; Thyroid Nodule; Ultrasonography
Background
Due to the significant improvement of high-frequency ultrasound and its wide application, the thyroid nodule diagnosis rate has increased from 19% to 68% in adults; among these, approximately 5–15% are malignant [1,2], accounting for more than 80% of all thyroid cancers. Papillary thyroid carcinoma (PTC) is the most common type of thyroid malignancy. Fortunately, PTC shows an indolent clinical course and extremely low mortality [3–6]. Thus, the major clinical challenge lies in distinguishing between PTC and benign nodules. Overdiagnosis may increase the number of unnecessary surgeries and costs for individuals, communities, and societies.
Various risk stratification systems for thyroid nodules based on US features developed by many different authors and societies can be used to improve the diagnostic accuracy of thyroid nodules. Features such as hypoechogenicity, irregular margins, taller-than-wide shape, central vascularity, and microcalcifications were widely regarded as suspicious ultrasound characteristics of malignant thyroid nodules [7–9]. However, fine-needle aspiration (FNA) cytology results rather than histopathologic results were applied in those risk stratification systems, and few of these studies involved Chinese populations.
It was documented that the rates of recurrence and distant metastasis in certain cases of PTC were 5–20% and were especially high in real-world studies of Chinese populations [10,11]. Cervical lymph node metastasis occurs in approximately 30–90% of PTCs [12]. Ultrasonography is one of the most sensitive screening methods for metastatic lymph nodes [13] and is widely used in clinical practice. Major ultrasound characteristics suggesting metastasis include the absence of an echogenic hilum, cystic change, presence of calcification, hyperechogenicity, round shape, and increased vascularization [14,15].
To the best of our knowledge, few published series has applied US features of thyroid nodules and cervical lymph nodes metastasis to distinguish between PTC and thyroid nodules. In this study, we aimed to develop an accurate and practical logistic regression model to distinguish papillary thyroid carcinomas from thyroid nodules in the early period of development in a Chinese population.
Material and Methods
Patients
The exception to the requirement of informed consent was approved by the appropriate institutional review board. We retrospectively collected clinical data from 1119 patients who underwent thyroidectomy in the First Affiliated Hospital of Nanjing Medical University (Nanjing, China) from January 2017 to January 2018. Postoperative histopathologic results, as the criterion standard, were verified by experts experienced in thyroid pathology. Among the 1119 nodules examined, 9 were excluded because they were determined to be medullary thyroid carcinoma (MTC, n=4) or follicular thyroid carcinoma (FTC, n=5) at postoperative pathological examination (Figure 1). This study included 1110 thyroid nodules within a population of 1110 patients. This study was approved by the Research Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (2018-SR-313).
Figure 1.

Flow chart of study participants.
Thyroid ultrasonography (US)
The 1110 preoperative thyroid ultrasonographic images were each re-analyzed by an experienced professional. The assessed features included the internal composition (solid or not), echogenicity (hypoechoic or not), margin (well-defined, spiculate, or ill-defined), border (indefinite or definite), capsular invasion, calcification status (micro, macro, mixed, or none), shape (irregular or regular), taller-than-wide shape,vascularity (central or not), and cervical lymph nodes metastasis features (absence of an echogenic hilum, cystic change, presence of calcification, hyperechogenicity, round shape, and increased vascularization) [7,8,14,15].
Statistical analysis
Statistical analyses were performed using SPSS version 20.0 (SPSS, Inc., Chicago, IL). Descriptive statistics are presented as the means±standard deviations for continuous variables and as the number of patients for categorical variables. Categorical data were analyzed using Pearson’s chi-square test or Fisher’s exact test, in which a p-value of <0.05 was considered statistically significant. Then, we used backward stepwise logistic regression to establish a model to differentiate PTC from benign thyroid nodules, applying a 10% significance level for removal. The sensitivity, specificity, accuracy, positive predictive value, and negative predictive value were calculated to demonstrate validity. A receiver operator characteristic curve (ROC) was drawn to assess the logistic regression model’s prediction performance.
Results
Clinical characteristics
We analyzed 1110 thyroid nodules from 1110 patients (845 women and 265 men, mean age, 44.6±12.7 years; range, 14–80 years). Among them, 849 patients (76.5%) had PTC and 261 (23.5%) had benign lesions. Surgery pathological findings confirmed that 223 patients (26.3%) were diagnosed with PTC with cervical lymph node metastasis. US examination reported 216 patients with suspicious lymph nodes with at least 1 metastatic feature. There were 557 patients 45 years old or older, including 376 with PTC and 181 with benign nodules. Of the 553 patients who were younger than 45 years old, 473 had PTC and 80 had benign nodules. All the nodules were confirmed by surgeries. Among benign nodules, the diagnosis included nodular goiter (n=192), thyroid adenoma (n=44), and Hashimoto’s nodule (n=25).
Age 45 years and age 55 years as the cutoff had statistical significance in distinguishing PTCs from benign nodules (p<0.000), but sex was not found to differ significantly between patients with PTCs and those with benign nodules (p=0.828) (Table 1).
Table 1.
Clinical characteristics of patients with thyroid nodules.
| Benign (n=261) | PTC (n=849) | P value | |
|---|---|---|---|
| Gender | |||
| Woman (base) | 200 | 645 | |
| Man | 61 | 204 | 0.828 |
| Age, y | |||
| ≥45(base) | 181 | 376 | |
| <45 | 80 | 473 | 0.000 |
| Age, y | |||
| ≥55 (base) | 98 | 156 | |
| <55 | 163 | 693 | 0.000 |
PTC – papillary thyroid carcinoma, P<0.05 was considered significant.
Sonographic features
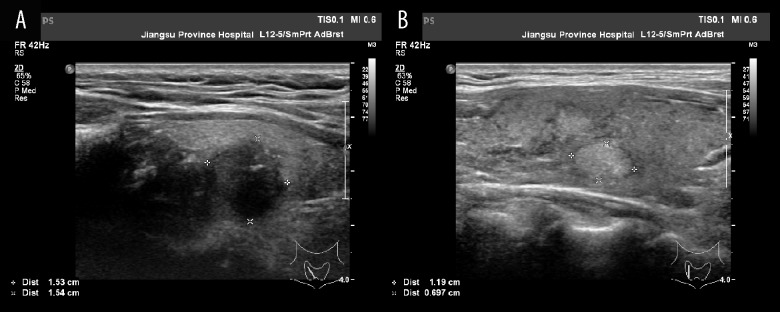
US features included the echogenicity (hypoechoic or not), margin (well-defined, spiculate, or ill-defined), border (indefinite or definite), shape (irregular or regular), taller-than-wide shape, capsular invasion, internal composition (solid or not), calcification status (micro, macro, mixed, or none), vascularity (central or not), and cervical lymph nodes metastasis features (absence of an echogenic hilum, cystic change, presence of calcification, hyperechogenicity, round shape, and increased vascularization) (Figure 2).
Figure 2.
(A) Papillary thyroid carcinoma: hypoechogenicity, ill-defined margin, taller than wide, microcalcification. (B) Benign nodule: hyperechogenicity, well-defined margin, definite border, taller than wide <1, no calcification.
Among the sonographic features, we found statistically significant differences between PTCs and benign nodules in terms of echogenicity (p<0.000), margin (p<0.000), border (p<0.000), shape (p<0.000), taller-than-wide shape (p<0.000), capsular invasion (p<0.000), internal composition (p<0.000), calcification status (p<0.000), vascularity (p<0.000), absence of an echogenic hilum (p<0.000), presence of calcification (p<0.000), hyperechogenicity (p<0.000), and increased vascularization (p=0.000) in cervical lymph nodes (Table 2).
Table 2.
US features of thyroid nodules and Cervical lymph nodes.
| Benign (n=261) | PTC (n=849) | P value | |
|---|---|---|---|
| Thyroid nodules | |||
| Echogenicity | 0.000 | ||
| Not hypoechoic | 108 | 34 | |
| Hypoechoic | 153 | 815 | |
| Margin | 0.000 | ||
| Well-defined | 218 | 314 | |
| Spiculate | 35 | 480 | |
| Ill-defined | 8 | 55 | |
| Border | 0.000 | ||
| Definite | 212 | 437 | |
| Indefinite | 49 | 412 | |
| Shape | 0.000 | ||
| Regular | 235 | 490 | |
| Irregular | 26 | 359 | |
| Taller than wide | 0.000 | ||
| ≤1 | 229 | 488 | |
| >1 | 32 | 361 | |
| Capsular invasion | 0.000 | ||
| No | 259 | 710 | |
| Yes | 2 | 139 | |
| Internal composition | 0.000 | ||
| Not solid | 102 | 50 | |
| Solid | 159 | 799 | |
| Calcification | 0.000 | ||
| No calcification | 145 | 140 | |
| Microcalcification | 90 | 658 | |
| Macrocalcification | 22 | 22 | |
| Mixed | 4 | 29 | |
| Vascularity | 0.000 | ||
| Not central | 152 | 286 | |
| Central | 109 | 563 | |
| Cervical lymph nodes | |||
| Absence of an echogenic hilum | 0.000 | ||
| No | 256 | 720 | |
| Yes | 5 | 129 | |
| Cystic change | 0.059 | ||
| No | 260 | 831 | |
| Yes | 1 | 18 | |
| Presence of calcification | 0.000 | ||
| No | 256 | 726 | |
| Yes | 5 | 123 | |
| Hyperechogenicity | 0.000 | ||
| No | 257 | 759 | |
| Yes | 4 | 90 | |
| Round shape | 0.080 | ||
| No | 261 | 837 | |
| Yes | 0 | 12 | |
| Increased vascularization | 0.000 | ||
| No | 258 | 759 | |
| Yes | 3 | 90 | |
PTC – papillary thyroid carcinoma, P<0.05 was considered significant.
A mathematical model
Logistic regression analysis confirmed that age under 45 years (OR=2.22, p=0.00), hypoechogenicity (OR=3.70, p=0.000), irregular shape (OR=2.13, p=0.004), ill-defined margin (OR=2.26, p=0.08), spiculate margin (OR=3.30, p=0.000), indefinite border (OR=2.45, p=0.000), capsular invasion (OR=7.76, p=0.006), taller-than-wide shape (OR=2.94, p=0.000), solid structure (OR=2.46, p=0.001), microcalcifications (OR=3.92, p=0.000), coexistence of microcalcifications and macrocalcifications (OR=5.84, p=0.006), and central vascularity (OR=2.10, p=0.001) were independently associated with increased risks for PTC, as well as lymph nodes metastasis features, including absence of an echogenic hilum (OR=3.74, p=0.027) and increased vascularization (OR=3.55, p=0.086) (Table 3).
Table 3.
logistic regression of risk factors for PTC.
| β | OR | 95%CI of OR | P value | |
|---|---|---|---|---|
| Age, y (x1) | ||||
| <45 | 0.80 | 2.22 | 1.48–3.35 | 0.000 |
| Echogenicity | ||||
| Hypoechoic | 1.31 | 3.70 | 2.08–6.56 | 0.000 |
| Margin | ||||
| Spiculate | 1.19 | 3.30 | 2.03–5.37 | 0.077 |
| Ill-defined | 0.82 | 2.26 | 0.92–5.58 | 0.000 |
| Border | ||||
| Indefinite | 0.90 | 2.45 | 1.58–3.82 | 0.000 |
| Shape | ||||
| Irregular | 0.76 | 2.13 | 1.28–3.57 | 0.004 |
| Taller than wide | ||||
| Yes | 1.08 | 2.94 | 1.78–4.85 | 0.000 |
| Capsular invasion | ||||
| Yes | 2.05 | 7.76 | 1.82–33.04 | 0.006 |
| Internal composition | ||||
| Solid | 0.90 | 2.46 | 1.42–4.27 | 0.001 |
| Calcification | ||||
| Microcalcification | 1.37 | 3.92 | 2.52–6.09 | 0.000 |
| Macrocalcification | 0.08 | 1.09 | 0.45–2.60 | 0.855 |
| Mixed | 1.76 | 5.84 | 1.67–20.37 | 0.006 |
| Vascularity | ||||
| Central | 0.74 | 2.10 | 1.37–3.21 | 0.001 |
| Cervical lymph nodes | ||||
| Absence of an echogenic hilum | ||||
| Yes | 1.32 | 3.74 | 1.16–12.06 | 0.027 |
| Increased vascularization | ||||
| Yes | 1.27 | 3.55 | 0.84–15.11 | 0.086 |
The model is a stepwise (backward selection) multivariable binary logistic model, applying a 10% significance level for removal. CI –confidence interval; OR – odds ratio.
The logistic regression model was established as follows:
Logit(P)=−3.75+0.80*age under 45 years+1.31*hypoechogenicity+ 0.76*irregular shape+0.82*ill-defined margin+1.19*spiculate margin+0.90*indefinite border+2.05*capsular invasion+ 1.08*taller-than-wide shape+0.90*solid structure+1.37*microcalcifications+1.76*coexistence of microcalcifications and macrocalcifications+ 0.74*central vascularity+1.32*absence of an echogenic hilum+1.27*increased vascularization.
The sensitivity, specificity, accuracy, positive predictive value, and negative predictive value of the model were 96.11%, 65.90%, 89.01%, 90.17%, and 83.90%, respectively. The ROC curve is shown in Figure 3 and the area under the curve (AUC) for this logistic regression model was 0.916. The best cutoff value for prediction was 0.75, achieving sensitivity and specificity of 86.9% and 81.2%, respectively.
Figure 3.

ROC curve for PTC prediction with a discrimination accuracy (AUC) of 0.916, 95%CI 0.896–0.936.
Discussion
The wide application of high-resolution ultrasound in clinical practice shows a significant increase in diagnose rate of thyroid nodules, which account for 5–15% of thyroid cancers, while papillary thyroid carcinoma (PTC) accounts about 85% of thyroid cancers.
Discriminating between PTCs and benign thyroid nodules is regarded as one of the most important challenges. In this study, we established an accurate and cost-effective model of distinguishing between PTC and benign thyroid nodules based on US features of thyroid nodules and cervical lymph nodes.
In this study, we used ages 45 and 55 years old as a cutoff point in predicting PTC, and in the end verified that age under 45 (OR=2.22, p=0.000) was an independent predictor of PTC. Patient age is closely related to prognosis in well-differentiated thyroid cancer (WDTC) and plays an important role in treatment [16].
For the past 2 decades, a patient age of 45 years was used as a cutoff point for demarcating age-associated survival in most of the major thyroid cancer staging systems [17,18]. In the eighth edition AJCC staging system for DTC, the age cutoff point was increased from 45 to 55 years to avoid low-risk patients being overstaged and overtreated [19]. The justification was based on studies of patients with both papillary (PTC) and follicular (FTC) thyroid carcinomas. In addition, few studies have examined the impacts of the ages in differentiating PTC from benign nodules. Based on our data, it is reasonable to regard age under 45 as an appropriate cutoff in this model.
Suspicious US features of malignant thyroid nodules are irregular margins, ill-defined, border, hypoechogenicity, microcalcification, taller-than-wide shape, and increased vascularity. Malignant-appearing PTCs were defined as those showing at least 1 suspicious US feature [8,20–22]. Suspicious US features differed slightly from those reported in previous studies. Consistent with previous findings, our study proved that the following characteristics could be risk factors for PTC: hypoechogenicity, irregular shape, ill-defined margin, spiculate margin, indefinite border, capsular invasion, taller-than-wide shape, solid structures, microcalcifications, coexistence of microcalcification and macrocalcifications, and central vascularity. However, most of these studies only considered the importance of US features of thyroid nodules and ignored the metastatic cervical lymph nodes in patients with papillary thyroid carcinoma. To avoid this, we analyzed the ultrasonographic characteristics of cervical lymph node metastasis. The absence of an echogenic hilum, cystic change, presence of calcification, hyperechogenicity, round shape, and increased vascularization were common findings typical of papillary carcinoma metastases [14,15,23].
In the present study, only the absence of an echogenic hilum (OR=3.74) and increased vascularization (OR=3.55) in lymph nodes had statistical significance in differentiating between PTC and benign nodules. The reasons for this finding could the following: 1) the distinction of calcifications and cystic change is difficult in certain sub-centimeter lymph nodes; 2) hyperechogenicity presents in both the adjacent muscles of benign nodules and in metastatic cervical lymph nodes; and 3) the sample size of PTC with metastatic cervical lymph nodes is too small. Thus, it remains controversial to apply these characteristics to distinguish benign from malignant lymph nodes, and further quantitative analysis is required.
Recently, some studies have reported several models for differentiating benign from malignant thyroid nodules in the Chinese population. Zhao et al. established a model based on the age, shape, blood flow distribution, and enhancement pattern for thyroid microcarcinoma. Contrast-enhanced ultrasound (CEUS) is not routinely performed in clinical practice in China [24]. Yongwen Zhang et al. established a diagnostic model using the risk factors of patient history, patient characteristics, physical examination, laboratory examination, and US features of thyroid nodules and cervical lymph nodes [25]. Jia Liu et al. retrospectively accessed clinical, laboratory, and US variables to established a mathematical model, the AUC of which is 0.808 [26].
In contrast to the smaller previous reports, our study enrolled 1110 patients and used the evidence from postoperative pathological results as the criterion standard instead of FNA cytology results. Additionally, our model is based on a single US examination aimed to improve the feasibility and practicality, achieving outstanding validity.
The accuracy, positive predictive value, and negative predictive value of the model were 89.01%, 90.17%, and 83.90%, respectively. The area under the curve (AUC) for this model was 0.916.
Our study has several limitations. First, this was a retrospective study and lacked randomization. A variety of nodules that were suspected to be benign may have been missed. Thus, sample selection bias may affect the validity of this model. Second, the data of this risk score diagnosis model came from patients who underwent thyroidectomy at a single center. Third, although the model is a significant improvement in diagnostic accuracy, the false-positive rate is still high (34.1%), which may have been caused by the following: (1) The results identified by histopathology of some misdiagnosed nodules were inflammatory lesions, the US characteristics of which are similar to PTCs; (2) Some typically benign thyroid nodules may experience morphologic changes over time, known as thyroid nodule mummification, including spontaneous shrinkage [27]. Hence, thyroid nodule mummification could be mistaken for malignant features of PTC on US examinations.
Conclusions
In summary, after analyzing a large number of thyroid ultrasound reports in routine clinical practice, the study confirmed the diagnostic value of a logistic regression model using US features of thyroid nodules and cervical lymph nodes for papillary carcinomas and benign nodules. Although there are many methods to deal with thyroid nodules, including ultrasound, laboratory tests, fine-needle aspiration (FNA), and molecular markers, US examination as a relatively cheap and simple tool will continue to be used most frequently in routine clinical practice. Therefore, our model is credible and cost-effective for PTC prediction. Large-scale and multicenter real-world studies are required to verify this model for PTC prediction.
Footnotes
Source of support: Key Research and Development programs of Jiangsu, China (Social Development – Clinical Frontier Technology) (BE2017736)
References
- 1.Guth S, Theune U, Aberle J, et al. Very high prevalence of thyroid nodules detected by high-frequency (13 MHz) ultrasound examination. Eur J Clin Invest. 2009;39:699–706. doi: 10.1111/j.1365-2362.2009.02162.x. [DOI] [PubMed] [Google Scholar]
- 2.Frates MC, Benson CB, Doubilet PM, et al. Prevalence and distribution of carcinoma in patients with solitary and multiple thyroid nodules on sonography. J Clin Endocrinol Metab. 2006;91:3411–17. doi: 10.1210/jc.2006-0690. [DOI] [PubMed] [Google Scholar]
- 3.Nam SY, Shin JH, Han BK, et al. Preoperative ultrasonographic features of papillary thyroid carcinoma predict biological behavior. J Clin Endocrinol Metab. 2013;98:1476–82. doi: 10.1210/jc.2012-4072. [DOI] [PubMed] [Google Scholar]
- 4.Ito Y, Miyauchi A, Kihara M, et al. Relationship between prognosis of papillary thyroid carcinoma patient and age: A retrospective single-institution study. Endocr J. 2012;59:399–405. doi: 10.1507/endocrj.ej12-0044. [DOI] [PubMed] [Google Scholar]
- 5.Ahn HS, Kim HJ, Welch HG. Korea’s thyroid-cancer “epidemic” – screening and overdiagnosis. N Engl J Med. 2014;371:1765–67. doi: 10.1056/NEJMp1409841. [DOI] [PubMed] [Google Scholar]
- 6.Brenner H. Long-term survival rates of cancer patients achieved by the end of the 20th century: A period analysis. Lancet. 2002;360:1131–35. doi: 10.1016/S0140-6736(02)11199-8. [DOI] [PubMed] [Google Scholar]
- 7.Tessler FN, Middleton WD, Grant EG, et al. ACR thyroid imaging, reporting and data system (TI-RADS): White paper of the ACR TI-RADS committee. J Am Coll Radiol. 2017;14:587–95. doi: 10.1016/j.jacr.2017.01.046. [DOI] [PubMed] [Google Scholar]
- 8.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer The American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perros P, Boelaert K, Colley S, et al. Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf) 2014;81(Suppl 1):1–122. doi: 10.1111/cen.12515. [DOI] [PubMed] [Google Scholar]
- 10.Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 11.Pellegriti G, Scollo C, Lumera G, et al. Clinical Behavior and outcome of papillary thyroid cancers smaller than 1.5 cm in diameter: Study of 299 cases. J Clin Endocrinol Metab. 2004;89:3713–20. doi: 10.1210/jc.2003-031982. [DOI] [PubMed] [Google Scholar]
- 12.Choi YJ, Yun JS, Kook SH, et al. Clinical and imaging assessment of cervical lymph node metastasis in papillary thyroid carcinomas. World J Surg. 2010;34:1494–99. doi: 10.1007/s00268-010-0541-1. [DOI] [PubMed] [Google Scholar]
- 13.do Rosario PW, Fagundes TA, Maia FF, et al. Sonography in the diagnosis of cervical recurrence in patients with differentiated thyroid carcinoma. J Ultrasound Med. 2004;23:915–20. doi: 10.7863/jum.2004.23.7.915. quiz 921–22. [DOI] [PubMed] [Google Scholar]
- 14.Leenhardt L, Erdogan MF, Hegedus L, et al. 2013 European thyroid association guidelines for cervical ultrasound scan and ultrasound-guided techniques in the postoperative management of patients with thyroid cancer. Eur Thyroid J. 2013;2:147–59. doi: 10.1159/000354537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosario PWS, de Faria S, Bicalho L, et al. Ultrasonographic differentiation between metastatic and benign lymph nodes in patients with papillary thyroid carcinoma. J Ultrasound Med. 2005;24:1385–89. doi: 10.7863/jum.2005.24.10.1385. [DOI] [PubMed] [Google Scholar]
- 16.Haymart MR. Understanding the relationship between age and thyroid cancer. Oncologist. 2009;14:216–21. doi: 10.1634/theoncologist.2008-0194. [DOI] [PubMed] [Google Scholar]
- 17.Shah JP, Loree TR, Dharker D, et al. Prognostic factors in differentiated carcinoma of the thyroid gland. Am J Surg. 1992;164:658–61. doi: 10.1016/s0002-9610(05)80729-9. [DOI] [PubMed] [Google Scholar]
- 18.Sherman SI, Brierley JD, Sperling M, et al. Prospective multicenter study of thyroiscarcinoma treatment: Initial analysis of staging and outcome. National Thyroid Cancer Treatment Cooperative Study Registry Group. Cancer. 1998;83:1012–21. doi: 10.1002/(sici)1097-0142(19980901)83:5<1012::aid-cncr28>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 19.Amin MBES, Greene F, Byrd DR, et al. AJCC Cancer Staging Manual. Eighth edition. New York, NY: Springer International Publishing; 2017. [Google Scholar]
- 20.Remonti LR, Kramer CK, Leitao CB, et al. Thyroid ultrasound features and risk of carcinoma: A systematic review and meta-analysis of observational studies. Thyroid. 2015;25:538–50. doi: 10.1089/thy.2014.0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimura H, Haraguchi K, Hiejima Y, et al. Distinct diagnostic criteria for ultrasonographic examination of papillary thyroid carcinoma: A multicenter study. Thyroid. 2005;15:251–58. doi: 10.1089/thy.2005.15.251. [DOI] [PubMed] [Google Scholar]
- 22.Moon HJ, Kwak JY, Kim MJ, et al. Can vascularity at power Doppler US help predict thyroid malignancy? Radiology. 2010;255:260–69. doi: 10.1148/radiol.09091284. [DOI] [PubMed] [Google Scholar]
- 23.Ahuja AT, Chow L, Chick W, et al. Metastatic cervical nodes in papillary carcinoma of the thyroid: Ultrasound and histological correlation. Clin Radiol. 1995;50:229–31. doi: 10.1016/s0009-9260(05)83475-0. [DOI] [PubMed] [Google Scholar]
- 24.Zhao RN, Zhang B, Yang X, et al. Logistic regression analysis of contrast-enhanced ultrasound and conventional ultrasound characteristics of sub-centimeter thyroid nodules. Ultrasound Med Biol. 2015;41:3102–8. doi: 10.1016/j.ultrasmedbio.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 25.Zhang YW, Meng FR, Hong LQ, Chu LF. A risk score model for evaluation and management of patients with thyroid nodules. Horm Metab Res. 2018;50:543–50. doi: 10.1055/a-0630-5239. [DOI] [PubMed] [Google Scholar]
- 26.Liu J, Zheng DM, Li Q, et al. A predictive model of thyroid malignancy using clinical, biochemical and sonographic parameters for patients in a multi-center setting. BMC Endocr Disord. 2018;18:17. doi: 10.1186/s12902-018-0241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lacout A, Chevenet C, Marcy PY. Mummified thyroid syndrome. Am J Roentgenol. 2016;206:837–45. doi: 10.2214/AJR.15.15267. [DOI] [PubMed] [Google Scholar]