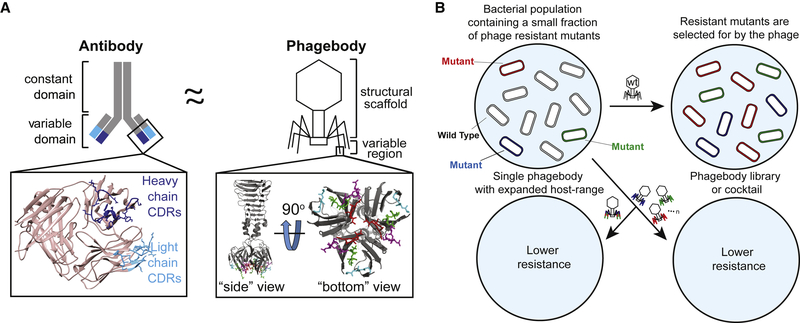
Figure 1. Phagebody Design and Proposed Ability to Target Bacterial Mutants.
(A) Schematic illustrating the similarities between antibodies and the phagebody tail fiber. In an antibody, antigen recognition is primarily encoded by six hypervariable complementarity-determining regions (CDRs), three located on the heavy chain and three on the light chain. The inset (left) presents the three-dimensional structure of the variable domain of an antibody (PDB: 1IGT) (Harris et al., 1997). Heavy chain CDRs are colored dark blue and light chain CDRs are colored teal. In phage T7, host-range is largely determined by the C terminus of its tail fiber protein, gp17. The inset (right) shows the crystallographic structure of the C-terminal 182 amino acids of T7 gp17 (PDB: 4A0T). Outward loops (red, magenta, green, and light blue) are expected to participate in receptor recognition while tolerating mutations. Phagebodies are designed to carry mutations in these loops while leaving other structures of the tail fiber intact.
(B) Schematic illustrating how resistance appears in bacterial cultures and how phagebody cocktails or individual phagebodies are proposed to suppress resistance.