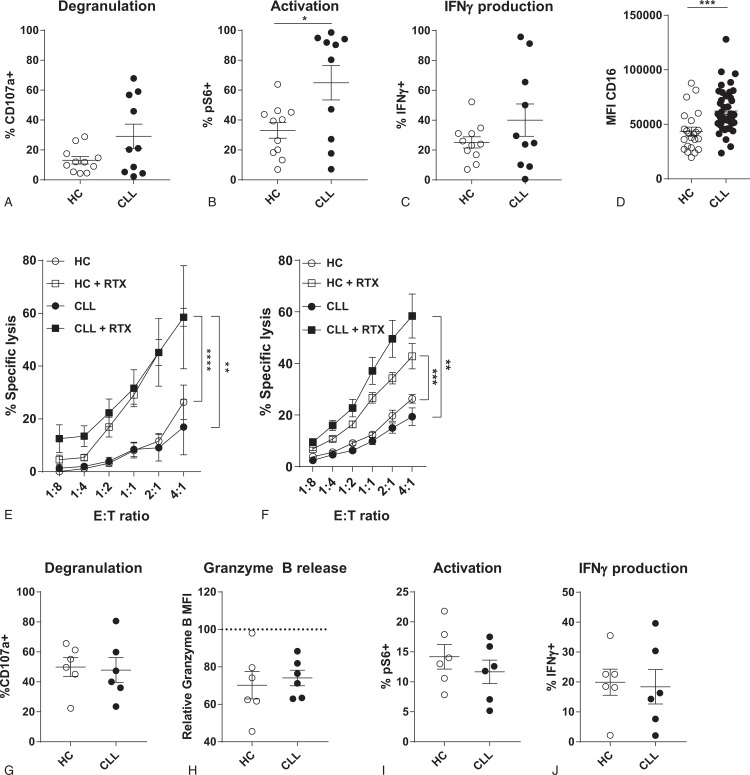
Figure 5.
CLL-derived NK cells are not intrinsically defective and can respond to opsonized target cells. (A–C) HC- or CLL-derived NK cells were stimulated with PMA/Ionomycin for 4 hours. Afterwards, degranulation (A), activation (B) and IFNγ production (C) were measured by flow cytometry. (D-H) HC- or CLL-derived NK cells were co-cultured with untreated or rituximab pre-treated Daudi cells in indicated E:T ratio's (D), or in a ratio of 1:10 (E-H) for 3 to 4 hours. Daudi cells were opsonized by culturing with 10 μg/ml rituximab for 30 minutes at 37°C. Target cell lysis and NK cell function were analyzed by flow cytometry. (D) Expression of CD16 on HC- and CLL-derived NK cells, analyzed by flow cytometry. (E) Percentage specific target cell lysis induced by HC- (open symbols) or CLL-derived (filled symbols) NK cells towards Daudi cells. (F) Percentage specific target cell lysis induced by HC- (open symbols) or CLL-derived (filled symbols) NK cells towards MEC1 cells with and without rituximab opsonization. (G) Percentage of degranulated (CD107a+) NK cells after co-culture with rituximab treated Daudi cells. (H) Release of granzyme B of NK cells after co-culture with rituximab treated Daudi cells. (I) Activation of NK cells after co-culture with rituximab treated Daudi cells. (J) Percentage of IFNγ producing NK cells after co-culture with rituximab treated Daudi cells. Bars indicate mean + SEM. ∗∗p < 0.01, ∗∗∗∗p < 0.0001 (One-Way ANOVA and Paired t test).