Abstract
Objectives
In 2017, Myanmar implemented routine viral load (VL) monitoring for assessing the response to antiretroviral therapy (ART) among people living with HIV (PLHIV). The performance of routine VL testing and implementation challenges has not yet assessed. We aimed to determine the uptake of VL testing and factors associated with it among PLHIV initiated on ART during 2017 in ART clinics of Yangon region and to explore the implementation challenges as perceived by the healthcare providers.
Design
An explanatory mixed-methods study was conducted. The quantitative component was a cohort study, and the qualitative part was a descriptive study with in-depth interviews.
Setting
Six ART clinics operated by AIDS/sexually transmitted infection teams under the National AIDS Programme.
Primary outcome measures
(1) The proportion who underwent VL testing by 30 March 2019 and the proportion with virological suppression (plasma VL <1000 copies/mL); (2) association between patient characteristics and ‘not tested’ was assessed using log binomial regression and (3) qualitative codes on implementation challenges.
Results
Of the 567 PLHIV started on ART, 498 (87.8%) retained in care for more than 6 months and were eligible for VL testing. 288 (57.8%, 95% CI: 53.3% to 62.2%) PLHIV underwent VL testing, of which 263 (91.3%, 95% CI: 87.1% to 94.4%) had virological suppression. PLHIV with WHO clinical stage 4 had significantly higher rates of ‘not being tested’ for VL. Collection of sample for VL testing only twice a month, difficulties in sample collection and transportation, limited trained workforce, wage loss and out-of-pocket expenditure for patients due to added visits were major implementation challenges.
Conclusions
The VL test uptake was low, with only six out of ten PLHIV tested. The VL testing uptake needs to be improved by strengthening sample collection and transportation, adopting point-of-care VL tests, increasing trained workforce, providing compensation to patients for wage loss and travel costs for additional visits.
Keywords: Antiretroviral therapy, PLHIV, treatment adherence, virological suppression, SORT IT, operational research
Strengths and limitations of this study.
First study from Myanmar to assess the implementation of routine viral load (VL) testing among people living with HIV (PLHIV) managed directly under the national AIDS programme.
First study from Asia region to have had explored the potential reasons for deficiencies in the implementation of VL testing in a programmatic setting.
Less scope for selection bias as all PLHIV in all six antiretroviral therapy (ART) clinics which initiated ART in the Yangon region were included.
The sample size was not adequate for assessing the factors associated with unsuppressed VL among those who underwent VL testing.
The interviews were conducted only among healthcare providers and thus, the study failed to capture perspectives of PLHIV.
Introduction
HIV infection is a major global public health issue. Globally in 2017, there were an estimated 36.9 million people living with HIV (PLHIV) and 0.9 million died due to HIV-related causes.1 There has been a steady decline in HIV incidence and deaths over the last decade, mainly due to the increase in coverage of antiretroviral therapy (ART) for PLHIV. To accelerate the efforts in HIV control and end the HIV epidemic by 2030, the Joint United Nations programme on HIV/AIDS (UNAIDS) recommended the 90-90-90 targets in 2014.2 The third ‘90’ target was to achieve virological suppression (viral load (VL) of <1000 copies per mL of plasma) among 90% of the PLHIV those are on ART. This implied that all the PLHIV on ART care need to receive VL testing.
The WHO has recommended routine VL monitoring as the preferred method to monitor response to ART and early identification of treatment failure.3 VL monitoring was recommended as the alternative methods like immunological (CD4 count) and clinical staging were relatively less sensitive and specific in assessing response to ART.3 The studies have shown that routine VL monitoring with appropriate action based on the VL can reduce the morbidity and mortality, resistance to ART and improve the treatment outcomes of second-line ART.4–7 However, the access to VL testing was limited in many low-income and middle-income countries due to financial, logistical and human resource constraints in the national AIDS control programmes.8 9 The uptake of routine VL monitoring among the patients ranged from 3% to 95% in seven sub-Saharan countries.10 Studies reported that there were individual and programme level factors which influenced the uptake of VL testing by the patients.11 12
Myanmar, with an estimated 230 000 PLHIV, has the second-highest number of cases in the South East Asia region.13 The UNAIDS considers Myanmar as a ‘country of concern’ as it continues to display a high incidence of new HIV infections. In 2016, Myanmar had approximately 11 000 reported new HIV infections (approximately 30 new infections per day).14 In spite of improved coverage of ART, about 7800 died due to AIDS-related illness in year 2016.13
In line with 90-90-90 targets of UNAIDS, the National Strategic Plan on HIV/AIDS of Myanmar in 2016 proposed the ‘90’s target to be achieved by 2020. One of the ‘90’ target was to achieve virological suppression in 90% of the PLHIV on ART.14 The National AIDS programme (NAP) recommended routine VL testing for all the PLHIV suspending the previous recommendation of targeted VL testing (only those patients who have clinical and immunological failure on first-line ART were referred for VL testing by clinicians). The VL testing was recommended at 6th month and 12th month after ART initiation, and every 12th month after that.
A study conducted while targeted VL testing was in practice reported uptake of VL testing in about 34% of the PLHIV receiving care under the ‘Integrated HIV Care’ (IHC) programme.15 The VL testing uptake among those availing ART services at public health facilities remained unknown. Also, since implementation of routine VL testing, there has been no systematic assessment on uptake of VL testing. There is anecdotal evidence that the uptake of VL testing is poor due to programmatic challenges in adhering to routine VL monitoring protocol. However, there is a need to understand the challenges faced by the field staff in implementing routine VL testing. This information could provide insights on existing problems in the system and enable programme managers to take corrective actions to improve the uptake of VL testing. In this regard, we aimed to assess the uptake of VL test after initiation of ART, factors associated with not receiving VL tests and challenges in implementation of routine VL monitoring as perceived by healthcare providers in six ART clinics operated by AIDS/STI (STI, sexually transmitted infection) teams located in public health facilities of Yangon, Myanmar.
Methodology
Study design
This was a concurrent explanatory mixed-methods study with cohort study using secondary data as a quantitative component and a descriptive qualitative component.16
Study setting
General setting
Yangon region is in the southern part of Myanmar and has a population of about 7 million. Around 70% of the population resides in urban areas.17 The region has an estimated HIV prevalence of 0.9% among adults, higher than that of the estimated prevalence of Myanmar (0.57%). The region has a concentrated epidemic of HIV with high prevalence among HIV key populations like men who have sex with men (26.6%) and female sex workers (24.6%).
The ART coverage in the region is about 68%. The ART is initiated in six out of eight ART clinics operated by AIDS/STI teams of NAP and also in ART clinics located in 21 general hospitals. The two ART clinics operated by the AIDS/STI teams lack medical officers to initiate ART and thus provide follow-up to patients initiated on ART elsewhere. The ART clinics at the general hospitals are operated by the specialist physician and not by the AIDS/STI teams of the NAP.
Specific setting
HIV care provision by AIDS/STI teams
The ART clinics operated by the AIDS/STI teams function directly under NAP and provide comprehensive HIV prevention and care services. These are located in the dedicated rooms of selected peripheral public health facilities. The team consists of one doctor, two trained nurses, two laboratory technicians and one counsellor. Both public and private health facilities refer PLHIV to these ART clinics for ART initiation. Once the PLHIV is registered, demographic and clinical information is recorded in the patient-specific treatment card (white card). With adoption of a ‘test and treat’ policy, all the PLHIV are initiated on ART once tested positive. Once initiated on ART, the PLHIV visits the ART clinics once every 3 months for follow-up and drugs are dispensed for 3 months during each visit. The clinical and investigation details are updated in the white card during the follow-up visits.
Routine VL monitoring
The PLHIV on ART is supposed to undergo the first routine VL testing after 6 months of initiation of ART. The doctor prescribes routine VL testing during the follow-up visits. Till July 2018, PLHIV were referred to the national health laboratory (NHL) at Yangon for getting the VL test done. Later on, AIDS/STI teams are collecting the blood sample twice a month and are transporting it to NHL for the VL test. Thus, the PLHIV prescribed for the VL test during their follow-up visit are asked to make an additional visit to the ART clinic on a prefixed date for sample collection. The nurse is supposed to document the details of the date of referral for VL testing, reason for referral and the date of blood sample collection in the white card.
The ART number and details of the sample received are documented in the laboratory information management system (LIMS). The plasma sample is used to test VL at NHL using the Abbott m2000rt platform. The lower limit of detection for the Abbott Platform is 40 copies/mL.18 The machine is connected to the LIMS and automatically updates the test results.
The staff from AIDS/STI team also collects the hard copy results from NHL twice a month when they visit to drop the samples for testing. The nurse documents the VL test results in the white card and also in the VL register. In case, VL is unsuppressed, PLHIV is offered enhanced adherence counselling. The VL test is repeated within 3 months of first test and if the VL is unsuppressed during the repeat test, the patient is initiated on second-line ART regimen.
Study site
This study was carried out in all the six ART clinics operated by AIDS/STI teams (North Oakkalapa and Mingalartaungnyunt teams in East district, Thanlyin team in South district, Latha and Kyi Myint Daing teams in the West district and Insein team in North district) which had facilities to initiate ART. The ART clinics were spread across four districts of the Yangon region.
Study population
Quantitative
All PLHIV initiated on ART from January 2017 to December 2017 in six ART clinics operated by AIDS/STI centres of the Yangon region were included in the study.
Qualitative
The healthcare providers (n=19) involved in care provision through AIDS/STI teams were interviewed. This included two district team leaders, four medical officers, four nurses, four counsellors and five laboratory technicians. We purposively selected those who were the focal point for the provision of the VL testing in their respective ART centres.
Data variables, sources of data and data collection
Quantitative
Data on demographic and clinical characteristics at ART initiation like ART number, age, gender, employment status, literacy, marital status, WHO clinical staging, CD4 count, date of initiation of ART, ART regimen, last follow-up visit date, end of follow-up status, date of death, date of loss to follow-up, date of transfer out, referral for VL testing, date of referral were extracted from the white card. The pretested proforma was used to extract these details. Data extraction was done by the principal investigator and coinvestigator during March 2019.
The electronic database with ART number, sample collection date, shipment date, date of receipt at laboratory, date of VL testing and copies of VL detected of all the VL testing done in the NHL during January 2017 to March 2019 were obtained from the LIMS.
Operational definition
Eligible for VL testing: individual who has been on follow-up care in the same ART clinic for at least 6 months after initiating the ART.
VL testing done: individual who has got his first routine VL testing done within 30 March 2019 (censoring date).
VL suppression: individual who has VL copies less than 1000 per mL.
Qualitative
In-depth interviews were conducted face-to-face in the local language (Myanmar) by KKT and NSA (both are female medical doctors trained in qualitative research). The interviews were audiorecorded using a mobile audio recorder after obtaining consent from the participant. The interviewer, KKT was working as a team leader of the AIDS/STI team of East district and involved in programme implementation. Thus, NSA, who was not involved in programme implementation, conducted interviews in the East district. The interviews were conducted in the participant’s workplace. Interviews lasted for an average of 30 min (range 15–55 min). At the end of each interview, a debriefing was done to ensure member checking.
Data entry and analysis
Quantitative
Data collected in the proforma were double entered and validated using EpiData entry software (EpiData Association, Odense, Denmark). Data were analysed using Stata V.12.0. The two electronic databases (EpiData and LIMS) were merged using ART number as a common identifier. The merged database was used for further analysis.
Sociodemographic, clinical and treatment characteristics were summarised using percentages. The eligible who received routine VL testing and who had VL suppression were summarised as percentages with 95% CI.
The binomial regression was used to assess the association between sociodemographic and clinical characteristics with not receiving VL testing. The unadjusted relative risks (RR) with 95% CI were calculated as measure of association. The median and IQR of duration at each step from sample collection to VL testing was calculated.
Qualitative
The interviewers prepared the transcripts using audiorecording on the same day of the interview. Transcription was done in Myanmar language. Manual descriptive content analysis was performed by two trained researchers (KKT and NSA) to generate codes and themes. The code and themes were translated into English. These were reviewed by the third reviewer (NTTK) to reduce subjectivity in analysis. The findings were reported by using ‘Consolidated criteria for Reporting Qualitative Research’ guidelines.19
Patient and public involvement
Principal investigator did not interact directly with the PLHIV registered for care in the ART clinics during this retrospective record review. The healthcare providers of AIDS/STI teams interviewed during the study enthusiastically participated. Findings from this study will help the NAP of Myanmar to identify the gaps and reasons for deficiency in routine VL testing and eventually improve the care and treatment of PLHIV.
Results
During the study reference period, 567 PLHIV were initiated on ART. The mean (SD) age of the PLHIV was 35.1 (10.5), and 325 (57.3%) were males. Of the total, 505 (89.1%) were literate, 398 (70.2%) were employed and 283 (49.9%) were currently married. During baseline evaluation at the start of ART, 236 (41.6%) were in stage 1 of the WHO clinical stage and 208 (36.7%) had a CD4 count of less than 200 per mm3. The sociodemographic and clinical characteristics of study participants are shown in table 1.
Table 1.
Sociodemographic and clinical characteristics of PLHIV initiated on ART in six ART clinics operated by AIDS/STI teams in Yangon Region during January to December 2017
| Characteristics | N (%)* |
| Total | 567 (100) |
| Age in years | |
| 15–29 | 190 (33.5) |
| 30–44 | 278 (49.0) |
| 45–59 | 90 (15.9) |
| 60 and above | 9 (1.6) |
| Gender | |
| Male | 325 (57.3) |
| Female | 242 (42.7) |
| Employment | |
| Yes | 398 (70.2) |
| No | 138 (24.3) |
| Not recorded | 31 (5.5) |
| Literate | |
| Yes | 505 (89.1) |
| No | 47 (8.3) |
| Not recorded | 15 (2.7) |
| Marital status | |
| Single | 148 (26.1) |
| Married | 283 (49.9) |
| Widow | 58 (10.2) |
| Divorced/separated | 57 (10.1) |
| Not recorded | 21 (3.7) |
| Clinic | |
| Clinic A | 272 (48.0) |
| Clinic B | 123 (21.7) |
| Clinic C | 21 (3.7) |
| Clinic D | 30 (5.3) |
| Clinic E | 39 (6.9) |
| Clinic F | 82 (14.5) |
| WHO clinical staging | |
| Stage 1 | 236 (41.6) |
| Stage 2 | 148 (26.1) |
| Stage 3 | 160 (28.2) |
| Stage 4 | 23 (4.1) |
| CD4 count (cells/mm3) | |
| 0–199 | 208 (36.7) |
| 200–349 | 114 (20.1) |
| 350–499 | 118 (20.8) |
| >500 | 116 (20.5) |
| Missing | 11 (1.9) |
| ART regimen | |
| TDF+3TC+EFV | 558 (98.4) |
| Other first line regimen | 9 (1.6) |
*Column percentage.
ART, antiretroviral therapy; CD4, cluster of differentiation 4/CD4+T helper cells; EFV, efavirenz; PLHIV, people living with HIV/AIDS; STI, sexually transmitted infection; 3TC, lamivudine; TDF, tenofovir.
After 6 months of initiation of ART, 498 (87.8%) retained in care and were eligible for routine VL testing. Among eligible, 288 (57.8%, 95% CI: 53.3% to 62.2%) got VL testing before censor date. The VL testing was done within 9 months of ART initiation in 56 (11.2%), whereas 119 (23.9%) were tested after 15 months. Among those who underwent routine VL testing, 263 (91.3%, 95% CI: 87.1% to 94.4%) had VL suppression. Of the 25 (8.7%) patients who had VL unsuppressed, eight (32.0%) had repeat VL testing after adherence counselling and six (75.0%) had unsuppressed VL on repeat test (figure 1).
Figure 1.
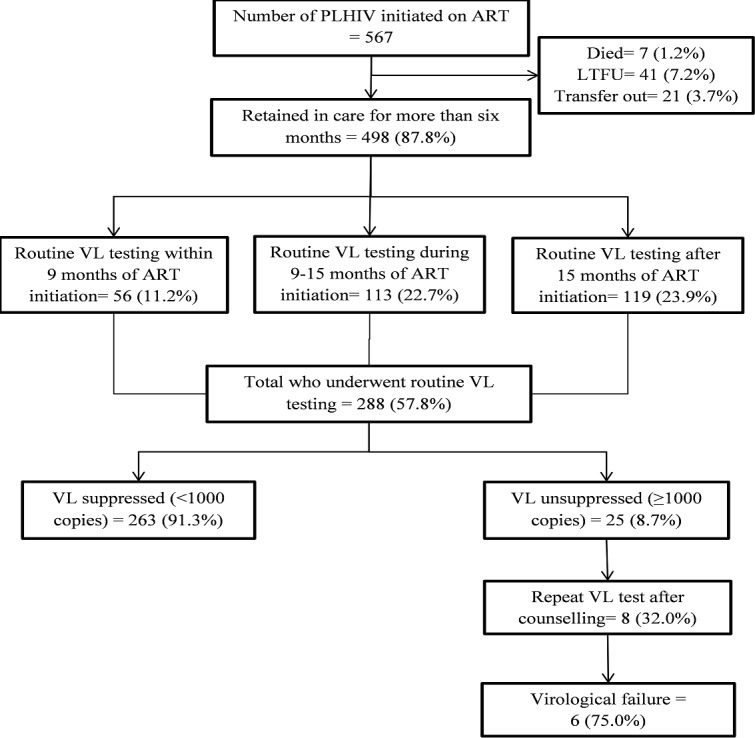
Flowchart depicting the uptake of VL testing and VL suppression among PLHIV initiated on ART in ART clinic operated by AIDS/STI teams in Yangon Region during 2017. ART, antiretroviral therapy; LTFU, loss to follow-up; PLHIV, people living with HIV/AIDS; STI, VL, viral load.
Table 2 shows the association between patient characteristics and not undergoing routine VL testing. Patients who received ART from clinic E (RR: 3.4, 95% CI: 1.4 to 8.5) and had WHO clinical stage 4 conditions at baseline (RR: 2.0, 95% CI: 1.5 to 2.7) had a higher risk of not undergoing routine VL testing.
Table 2.
Association of sociodemographic, clinical and treatment-related factors with not having at least one routine VL testing among those PLHIV initiated on art in art clinics operated by AIDS/STI teams in Yangon region during 2017
| Characteristics | Categories | Total, N (%) * | Not tested, N (%)† | Unadjusted RR (95% CI) | P value |
| Total | 498 (100) | 210 (42.2) | |||
| Age in years | 15–29 | 157 (31.5) | 71 (45.2) | 1.2 (0.9 to 1.7) | 0.212 |
| 30–44 | 250 (50.2) | 106 (41.6) | 1.1 (0.8 to 1.6) | 0.432 | |
| 45–59 | 82 (16.5) | 30 (36.6) | 1 | ||
| 60 and above | 9 (01.8) | 5 (55.6) | 1.5 (0.8 to 2.9) | 0.208 | |
| Gender | Male | 286 (57.4) | 125 (43.7) | 1.1 (0.9 to 1.3) | 0.422 |
| Female | 212 (42.6) | 85 (40.1) | 1 | ||
| Employment | Yes | 356 (71.5) | 151 (42.4) | 1 | |
| No | 115 (23.1) | 52 (45.2) | 1.1 (0.8 to 1.3) | 0.593 | |
| Not recorded | 27 (05.4) | 7 (25.9) | 0.6 (0.3 to 1.2) | 0.137 | |
| Literate | Yes | 443 (89.0) | 188 (42.4) | 1 | |
| No | 40 (08.0) | 18 (45.0) | 1.1 (0.7 to 1.5) | 0.749 | |
| Not recorded | 15 (03.0) | 4 (26.7) | 0.6 (0.3 to 1.4) | 0.282 | |
| Marital status | Single | 139 (27.9) | 53 (38.1) | 1.2 (0.8 to 1.9) | 0.447 |
| Married | 241 (48.4) | 110 (45.6) | 1.4 (0.9 to 2.2) | 0.096 | |
| Widow | 53 (10.6) | 17 (32.1) | 1 | ||
| Divorced/separated | 49 (09.8) | 23 (46.9) | 1.5 (0.9 to 2.4) | 0.129 | |
| Not recorded | 16 (03.2) | 7 (43.8) | 1.4 (0.7 to 2.7) | 0.371 | |
| Clinic | Clinic A | 234 (47.0) | 104 (44.4) | 2.3 (1.0 to 5.7) | 0.063 |
| Clinic B | 110 (22.1) | 49 (44.6) | 2.3 (0.9 to 5.8) | 0.066 | |
| Clinic C | 21 (04.2) | 4 (19.1) | 1 | ||
| Clinic D | 24 (04.8) | 9 (37.5) | 2.0 (0.7 to 5.5) | 0.194 | |
| Clinic E | 34 (06.8) | 22 (64.7) | 3.4 (1.4 to 8.5) | 0.009 | |
| Clinic F | 75 (15.1) | 22 (29.3) | 1.5 (0.6 to 4.0) | 0.373 | |
| WHO staging | Stage 1 | 208 (39.4) | 82 (39.4) | 1 | |
| Stage 2 | 57 (41.6) | 57 (41.6) | 1.1 (0.8 to 1.4) | 0.685 | |
| Stage 3 | 55 (41.4) | 55 (41.4) | 1.0 (0.8 to 1.4) | 0.722 | |
| Stage 4 | 16 (07.6) | 16 (80.0) | 2.0 (1.5 to 2.7) | <0.001 | |
| CD4 count (cells/mm3) | 0–199 | 176 (35.3) | 74 (42.1) | 1.2 (0.9 to 1.6) | 0.215 |
| 200–349 | 103 (20.7) | 50 (48.5) | 1.4 (1.0 to 1.9) | 0.050 | |
| 350–499 | 110 (22.1) | 38 (34.6) | 1 | ||
| >500 | 102 (20.5) | 45 (44.1) | 1.3 (0.9 to 1.8) | 0.155 | |
| Missing | 7 (01.4) | 3 (42.9) | 1.2 (0.5 to 3.0) | 0.636 | |
| ART regimen | TDF+3TC+EFV | 489 (98.2) | 206 (42.1) | 1 | |
| Other regimen | 9 (01.8) | 4 (44.4) | 1.1 (0.5 to 2.2) | 0.887 |
*Column percentage.
†Row percentage.
ART, antiretroviral therapy; CD4, cluster of differentiation 4/CD4+T helper cells; EFV, efavirenz; PLHIV, people living with AIDS/HIV; RR, relative risk; STI, sexually transmitted infection; 3TC, lamivudine; TDF, tenofovir; VL, viral load.
Table 3 shows the median duration at various stages from the collection of the blood sample to VL testing. The total median duration from sample collection to VL testing was 4 (IQR: 3–10) days. The duration between receipt of sample in the NHL and VL testing was 3 (IQR: 1–7) days.
Table 3.
Duration of various stages from sample collection to the reporting of viral load test result among those PLHIV who underwent routine viral load testing in Yangon region during 2017
| Stages | Median (IQR) |
| Sample collection to shipment | 0 (0–1) |
| Sample shipment to receipt at laboratory | 2 (0–7) |
| Receipt at laboratory to VL testing | 3 (1–7) |
| Total | 5 (3–10) |
PLHIV, people living with HIV/AIDS; VL, viral load.
Qualitative findings
Challenges in implementation of routine VL testing
Twenty-one codes were deduced from the transcripts and were categorised into three major categories. The categories and codes are summarised in figure 2 and briefly described.
Figure 2.
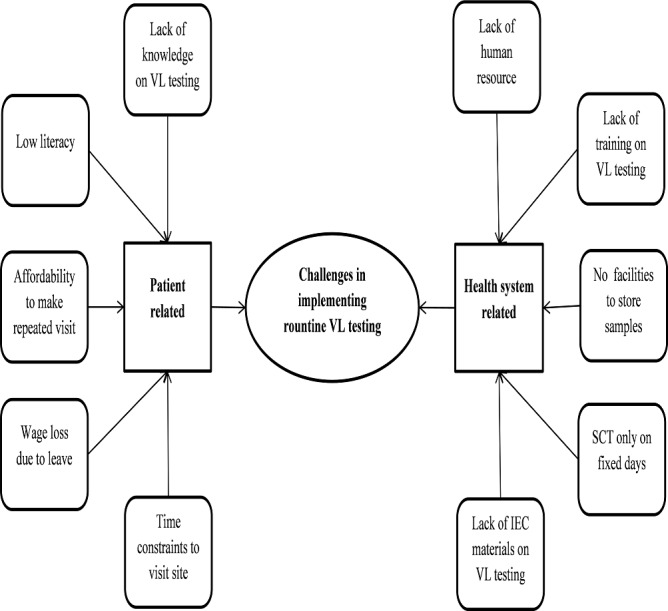
Concept map showing challenges for implementation of routine VL testing in AIDS/STI art clinics operated by AIDS/STI teams in Yangon region during 2017. IEC, information, education and communication; SCT, sample collection and transportation; STI, sexually transmitted infection; VL, viral load.
Patient-related challenges
Healthcare providers feel that there is a knowledge-gap between providers and patients about the importance of VL monitoring.
Patients are aware of CD4 but they do not know much about routine VL services, so we have to explain about VL testing and its importance. (Medical Officer)
The providers feel that the low level of education among patients as one of the barriers for educating and motivating patients for routine VL testing.
There are still many illiterate patients, so it is difficult to explain about our (VL) routine service. (Counsellor)
The patients willing to get the VL testing has to travel to ART clinics for sample collection. As the majority of patients are employed, require them to take leave for getting the services, they do not come for VL testing.
It is not because they are not willing to do the test, but because they cannot come as they are in formal employment (Medical officer)
Even when the patient reaches the sites for VL testing, few times due to increased workload in the sites, the sample collection is delayed. As patients have to wait for long hours, they go back without giving the sample.
Patients take half day leave for the testing, so they don’t want to wait for long. (ART Nurse)
Some patients choose the clinic far from them for their HIV treatment and care as they concern about the confidentiality of their HIV status. The poor patients living far from clinics could not afford travel costs to make an additional visit to the clinic for VL testing.
There are some patients who live far from the team as they don’t want to take ARVs (anti-retroviral) at the DC (decentralised ART) site near to their home; therefore they have to come not only for ART but also for necessary investigations……. Some poor patients need support for travel cost to come to the clinic. (Medical Officer)
Health system-related challenges
The sample collection for VL testing is offered only twice a month. This requires the patients to visit the ART clinic exclusively for getting the VL testing other than their regular ART follow-up visit. As the patients have to make repeated visits, they miss the appointments.
Sample collection is done on Wednesday, and ART regular follow up visit days are Monday, Wednesday and Friday. For patients who come to clinic on Wednesday, it is fine, for others we have to call them again for VL testing. In my experience, about 1/3 of patients could not come next time for blood sample collection. (Medical Officer)
The provider’s felt that there was a shortage of human resource and it was difficult to manage the sample collection and transportation for VL testing with the existing staff. The staffs from the clinic have to carry the samples to NHL. Thus, it required them to be out of the clinic on sample transportation days. This disturbed the routine care provision in the clinic.
We have limited HR to do the blood sample collection and transportation.… It is not feasible to do the sample collection and ART follow up at the same day currently. (District Team Leader)
Also, providers raised their concern about the lack of laboratory technicians who are involved mainly in VL testing service provision.
There is no lab technician at the team, the lab technician is attached from the hospital. We want lab technician for the team. (Medical officer)
Also, there were challenges in transporting the collected samples. The staff felt that the boxes provided for transportation of the samples were not comfortable to use.
Sample carrier (cold box) is too big to carry in my opinion, I want smaller one.
The staff felt that the transportation and collection sample disrupted their routine duties as the staff had to go in-person to collect results from the NHL.
We go to NHL to get the results, but few times we will not get all the results. We need to go to different sections within NHL to collect results on VL testing, CD4, and other biochemical tests (Laboratory Technician)
The providers felt there was a lack of training related to VL testing. Only a few in each facility were trained.
Only one nurse gets the training, among 2 nurses. I think all health workers of the team need training for VL. I did not get training for HIV viral load and want to get training. (ART nurse)
The providers felt there is a lack of information, education and communication (IEC) materials for educating the PLHIV on VL testing and its importance.
There is no pamphlet available for the viral load testing, we do explain (about VL) by ourselves. It will be better if the pamphlets are distributed. (ART Nurse)
The providers felt the VL testing had increased their workload with an increase in the paper-based documentation.
Unlike CD4 and other blood tests, we have to use separate VL register and requisition forms, it is an extra work for recording. (Medical officer)
Programmatic changes which have improved VL testing
The providers felt that the programmatic change to collect and transport the samples for VL testing instead of referring the patient to NHL for VL testing had improved the VL testing uptake.
In the past, we refered patients to NHL with VL requisition form, now patients have to come only to our team to do sample collection for VL. (Team Leader)
Discussion
To our knowledge, this is the first study from Myanmar assessing the uptake of VL testing among PLHIV after the introduction of routine VL monitoring under NAP in 2017. Also, the study explored the challenges in the implementation of routine VL monitoring in public health facilities. About six out of 10 PLHIV had at least one routine VL testing during the study follow-up period of 27 months. However, only one out of 10 patients got routine VL testing within 9 months of ART initiation, wherein the NAP guidelines recommended the first VL test at 6 months.20 The viral suppression rate among the patients who underwent VL testing was high. Only one-third of the patients with unsuppressed VL underwent repeated testing. The major challenges in implementing routine VL testing were the need for an additional visit for VL testing, inadequate knowledge about VL testing among patients, wage loss and expenditure on travel to receive VL test, limited human resources in facilities, lack of training and difficulties in sample transportation.
The current study had lower uptake of routine VL testing compared with those seen in Swaziland (87%), Rwanda (93.2%) and Indonesia (71.1%).21–23 As routine VL testing was introduced recently in 2017, the early implementation challenges and barriers such as the need for repeated visits only for VL sample collection would have led to lower uptake of VL testing. However, the NAP of Myanmar is responsive and has already implemented a few interventions to overcome the challenges and improve the uptake. The programme has recently outsourced the VL testing to private laboratories to negate the challenges in the transportation of samples to reference laboratories. Also, high-burden ART clinics have begun to collect samples once a week and transport them through peer volunteers. These programmatic changes could improve the uptake of VL testing. The increase in uptake over 6 months, 12 months and more than 15 months in the study might be due to the strengthening of blood sample collection and transport mechanisms.
The previous study from Myanmar had assessed the VL test uptake during VL testing through ‘targeted’ approach. Thus, the result cannot be compared. However, the uptake has improved after implementation of routine VL testing compared with 34% coverage reported from the IHC Programme, an integrated programme operated in collaboration with NAP, National TB Control Programme and the International Union Against Tuberculosis and Lung Disease (The Union) of Myanmar.15 24
Though there was a high VL suppression rate, it has to be interpreted with caution as it is derived from the PLHIV who underwent VL testing. The PLHIV received a VL test might have better treatment adherence compared with the large proportion of patients who did not undergo a test. Thus, the percentage of VL suppression might be an overestimate due to inherent selection bias. Similar to our study, the studies in the past have reported a high rate of viral suppression. However, these reported high rates could be due to the bias of deducing the percentage only among those who underwent VL test.22 23 25
The study has a few strengths. First, we used a mixed-methods design. The qualitative component brought out the important challenges in implementing routine VL testing, which might have led to lower uptake of VL testing. Second, the study used the routinely collected programme data and thus, reflects the ground realities. Third, PLHIV from all the ART clinics operated by AIDS/STI teams and initiated ART in the Yangon region were included and thus, there was less scope for selection bias.
There are several limitations. First, the study was conducted only in ART clinics operated by AIDS/STI teams in the Yangon region, where each team can reach the NHL within 2 hours. In few regions of Myanmar, the samples are transported to far off VL testing facilities. Considering the transportation of samples as the major challenge for uptake of VL testing, the study results cannot be generalised to other regions of Myanmar. Second, the investigators intended to extract the details like reason for referral for VL testing by the medical officer and date of referral from the white cards. However, it was not possible due to a deficiency in the recording of referral details under the programme. Thus, the proportion referred for VL testing and the proportion referred to but not undergoing a VL test were not determined. Third, the sample size was not adequate for assessing the factors associated with unsuppressed VL among those who underwent VL testing. Fourth, the potential factors like the economic status of the patient, distance to ART clinic and adherence to follow-up visits, which could be associated with uptake of VL testing, were not assessed as we relied only on secondary data. Fifth, the interviews were conducted only among healthcare providers and thus, the study failed to capture perspectives of PLHIV. However, the healthcare providers who were closely involved in patient care and had better insights on the challenges faced by the patients were interviewed.
The study has a few implications and recommendations. First, the uptake of routine VL testing is low and the majority does not undergo the test at 6 months as recommended by NAP. The scheduling of the sample collection for VL testing at ART clinics only once in 2 weeks and expecting patients to make an additional visit for VL testing was seen as a potential reason for low uptake.26 The programme needs to devise strategies to offer the VL sample collection during the patient’s regular follow-up visits. If samples are collected each day, either the ART clinic has to be equipped with storage facilities or the transportation mechanism has to be strengthened to send these samples to reference laboratories daily.27 Alternatively, the NAP can consider either the point-of-care (POC) VL test kits or dried blood spot samples for VL testing.28 These alternate options could improve the uptake, as the sample can be taken at patients’ and providers’ convenient time and it is easy to arrange for transportation.
Second, the existing staffs are involved in the transportation of the samples and this disturbs their routine patient care activities. The NGOs or incentivised peer volunteers can be utilised for the transportation of samples. Also, efficient sample transportation networks can be devised to reduce the delay and cost associated with transportation.29
Third, the healthcare providers feel that there is a lack of knowledge among PLHIV about VL testing and also, there are not enough IEC materials to educate patients. The programme needs to invest in VL demand generation activities through the involvement of patient care groups, NGOs working with PLHIV and peer volunteers/counsellors. Also, the provision of incentives to patients for having VL suppression could improve the ART adherence and uptake VL testing.
Fourth, the programme has to strengthen the recording and reporting of VL referral, test results and the actions taken if the patient has unsuppressed VL. The ‘team leaders’ need to routinely check the patient records for completeness in documentation of VL-related details during their supervisory visits.
Fifth, cohort-based indicators like ‘VL test uptake rate at 6 months’ can be added in monthly reporting formats of ART clinics for monitoring the performance of clinics in the provision of routine VL monitoring.
Conclusion
The VL test uptake was low, with only six out of 10 PLHIV tested. Collection of sample for VL testing only twice a month, difficulties in sample collection and transportation, limited trained workforce, wage loss and out-of-pocket expenditure for patients due to added visits were major implementation challenges. The VL test uptake needs to be improved by supporting sample collection and transportation, adopting POC VL tests, increasing trained workforce in clinics, providing compensation to patients for wage loss and travel costs for additional visits.
Supplementary Material
Acknowledgments
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO/TDR). The model is based on a course developed jointly by the International Union Against Tuberculosis and Lung Disease (The Union) and Medecins Sans Frontieres (MSF/Doctors Without Borders). The specific SORT IT programme which resulted in this publication was jointly organised and implemented by The Centre for Operational Research, The Union, Paris, France; Department of Medical Research, Ministry of Health and Sports, Yangon; Department of Public Health, Ministry of Health and Sports, Nay Pyi Taw; The Union Country Office, Mandalay, Myanmar; The Union South-East Asia Office, New Delhi, India and London School of Hygiene and Tropical Medicine, London, UK.
Footnotes
Contributors: KKT was the principal investigator; PT and NTTK were the SORT IT course mentors; SH and HNO are the senior authors. KKT, NSA, TMZ and PS were involved in data acquisition; KKT, PT and NTTK analysed the data and prepared the first draft of paper. All authors were involved in conception, design, inference of results, providing critical review to the manuscript and approval of the manuscript from this protocol.
Funding: The training programme, within which this paper was developed, was funded by the Department for International Development (DFID), London, UK.
Disclaimer: The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Competing interests: None declared.
Patient consent for publication: Informed written consent was obtained from study participants included in the qualitative study. The National AIDS programme permitted to extract the routine programme data.
Ethics approval: The study proposal was approved by Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease, Paris, France (EAG number-50/18) and Ethical Review Committee, Department of Medical Research, Yangon, Myanmar (Ethics/DMN20191030).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available in a public, open access repository.
Technical appendix, statistical code and data set available from the https://www.dropbox.com/sh/4vx3ktpfx6mgl1u/AADYD_p9UX-6naW1Gi__y-H2a?dl=0
References
- 1. World Health Organization HIV/AIDS fact sheet. World Health Organization, 2019. https://www.who.int/news-room/fact-sheets/detail/hiv-aids [Google Scholar]
- 2. Joint United Nations Programme on HIV/AIDS (UNAIDS) 90-90-90: an ambitious treatment target to help end the AIDS epidemic. Geneva, Switzerland, 2016. [Google Scholar]
- 3. World Health Organization Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations. Geneva, Switzerland, 2016. [PubMed] [Google Scholar]
- 4. Piot P, Quinn TC. Response to the AIDS pandemic – a global health model. N Engl J Med 2013;368:2210–8. 10.1056/NEJMra1201533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Estill J, Kerr CC, Blaser N, et al. . The effect of monitoring viral load and tracing patients lost to follow-up on the course of the HIV epidemic in Malawi: a mathematical model. Open Forum Infect Dis 2018;5:ofy092 10.1093/ofid/ofy092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sollis KA, Smit PW, Fiscus S, et al. . Systematic review of the performance of HIV viral load technologies on plasma samples. PLoS One 2014;9:e85869 10.1371/journal.pone.0085869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peter T, Ellenberger D, Kim AA, et al. . Early antiretroviral therapy initiation: access and equity of viral load testing for HIV treatment monitoring. Lancet Infect Dis 2017;17:e26–9. 10.1016/S1473-3099(16)30212-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization The use of antiretroviral drugs for treating and preventing HIV infection. 2nd edn Geneva, Switzerland, 2016. [PubMed] [Google Scholar]
- 9. Lecher S, Williams J, Fonjungo PN, et al. . Progress with scale-up of HIV viral load monitoring - seven sub-Saharan African countries, January 2015-June 2016. MMWR Morb Mortal Wkly Rep 2016;65:1332–5. 10.15585/mmwr.mm6547a2 [DOI] [PubMed] [Google Scholar]
- 10. Roberts T, Cohn J, Bonner K, et al. . Scale-Up of routine viral load testing in resource-poor settings: current and future implementation challenges. Clin Infect Dis 2016;62:1043–8. 10.1093/cid/ciw001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rutstein SE, Golin CE, Wheeler SB, et al. . On the front line of HIV virological monitoring: barriers and facilitators from a provider perspective in resource-limited settings. AIDS Care 2016;28:1–10. 10.1080/09540121.2015.1058896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Swannet S, Decroo T, de Castro SMTL, et al. . Journey towards universal viral load monitoring in Maputo, Mozambique: many gaps, but encouraging signs. Int Health 2017;9:206–14. 10.1093/inthealth/ihx021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Avert HIV and AIDS in MYANMAR-2018. avert, 2019. Available: https://www.avert.org/professionals/hiv-around-world/asia-pacific/myanmar
- 14. Ministry of Health and Sports National strategic plan on HIV and AIDS Myanmar 2016-2020. National AIDS Program, 2016. [Google Scholar]
- 15. Kyaw NTT, Harries AD, Kumar AMV, et al. . High rate of virological failure and low rate of switching to second-line treatment among adolescents and adults living with HIV on first-line art in Myanmar, 2005-2015. PLoS One 2017;12:e0171780 10.1371/journal.pone.0171780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Creswell J, Clark PV. Designing and conducting mixed methods research. London, United Kingdom: Sage Publications Ltd, 2007. [Google Scholar]
- 17. Ministry of labour of labour Immigration and Population The 2014 Myanmar population and housing census: Yangon region census report volume 3L (2015). Nay Pyi Taw: Department of Population, Ministry of Immigration and Population, 2014. [Google Scholar]
- 18. Nkeze J, Liang D, Adkins H, et al. . Comparison of HIV-1 viral load between Abbott m2000 and Roche Cobas TaqMan methods. J Antivir Antiretrovir 2010;02 10.4172/jaa.1000021 [DOI] [Google Scholar]
- 19. Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007;19:349–57. 10.1093/intqhc/mzm042 [DOI] [PubMed] [Google Scholar]
- 20. National AIDS Programme Myanmar The clinical management of hiv infection in myanmar guideline(fifth edition). J Chem Inf Model 2013;53:1689–99.23800267 [Google Scholar]
- 21. Jobanputra K, Parker LA, Azih C, et al. . Impact and programmatic implications of routine viral load monitoring in Swaziland. J Acquir Immune Defic Syndr 2014;67:45–51. 10.1097/QAI.0000000000000224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ndagijimana Ntwali JdeD, Decroo T, Ribakare M, et al. . Viral load detection and management on first line art in rural Rwanda. BMC Infect Dis 2019;19:8 10.1186/s12879-018-3639-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Januraga PP, Reekie J, Mulyani T, et al. . The cascade of HIV care among key populations in Indonesia: a prospective cohort study. The Lancet HIV 2018;5:e560–8. 10.1016/S2352-3018(18)30148-6 [DOI] [PubMed] [Google Scholar]
- 24. Phyo KH, Oo MM, Harries AD, et al. . High prevalence and incidence of tuberculosis in people living with the HIV in Mandalay, Myanmar, 2011-2017. Int J Tuberc Lung Dis 2019;23:349–57. 10.5588/ijtld.18.0436 [DOI] [PubMed] [Google Scholar]
- 25. Dat VQ, Duong BD, Nhan DT, et al. . Viral load suppression and acquired HIV drug resistance in adults receiving antiretroviral therapy in Viet Nam: results from a nationally representative survey. Western Pac Surveill Response J 2018;9:16–24. 10.5365/wpsar.2018.9.1.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pham MD, Romero L, Parnell B, et al. . Feasibility of antiretroviral treatment monitoring in the era of decentralized HIV care: a systematic review. AIDS Res Ther 2017;14:3 10.1186/s12981-017-0131-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nkengasong JN, Yao K, Onyebujoh P. Laboratory medicine in low-income and middle-income countries: progress and challenges. Lancet 2018;391:1873–5. 10.1016/S0140-6736(18)30308-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nichols BE, Girdwood SJ, Shibemba A, et al. . Cost and impact of dried blood spot versus plasma separation card for scale-up of viral load testing in resource-limited settings. Clin Infect Dis 2019:ciz338 10.1093/cid/ciz338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nichols BE, Girdwood SJ, Crompton T, et al. . Impact of a borderless sample transport network for scaling up viral load monitoring: results of a geospatial optimization model for Zambia. J Int AIDS Soc 2018;21:e25206 10.1002/jia2.25206 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


