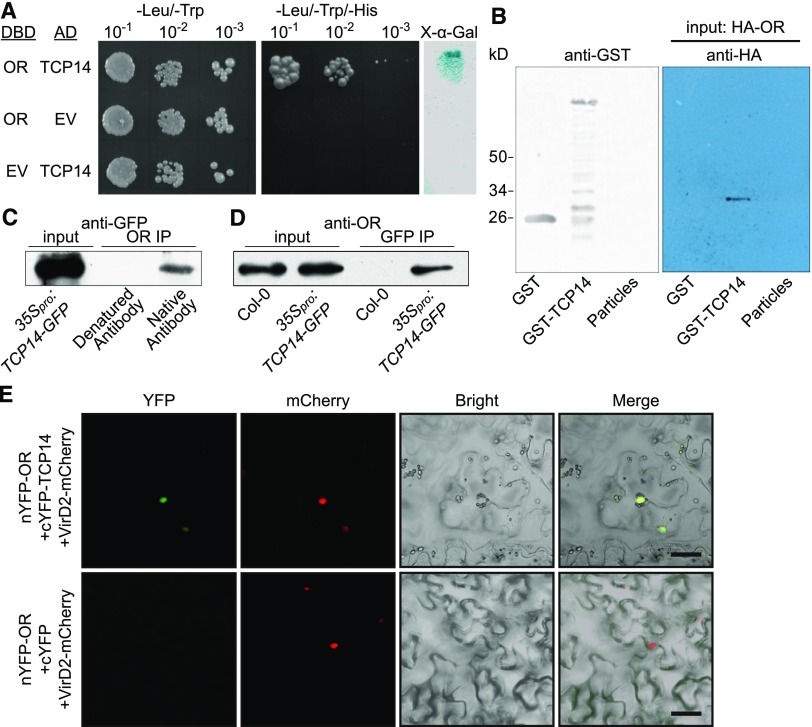
Figure 5.
OR Interacts with TCP14 in the Nucleus.
(A) Yeast two-hybrid assay. TCP14 was cloned into pDEST22, which encodes the activation domain, and OR was cloned into pDEST32, which encodes the DNA binding domain (DBD). Yeast AH109 cells were cotransformed with a combination of the indicated plasmids or empty vector and plated onto nonselective (−Leu/−Trp) and selective (−Leu/−Trp/−His) plates. A colony-lifting assay with 5-bromo-4-chloro-3-indolyl-α-d-galactopyranoside as the substrate was performed to confirm the interaction (right). AD, activation domain; EV, empty vector; X-α-Gal, 5-bromo-4-chloro-3-indolyl-α-d-galactopyranoside.
(B) Pull-down assay. GST-TCP14 fusion protein, GST, and glutathione particles were incubated with HA-OR that was translated in a wheat germ system. The bound protein was eluted, resolved by SDS-PAGE, blotted, and probed with the antibody against the HA tag.
(C) and (D) Co-IP assays using total protein extracted from the 35Spro:TCP14-GFP seedlings. (C) Co-IP using the antibody against ORcTP. The captured proteins were probed with the anti-GFP antibody to detect the presence of TCP14-GFP fusion protein that co-precipitated with OR. Denatured antibody against ORcTP was used as a negative control. (D) Co-IP using the anti-GFP antibody. The captured proteins were probed with the antibody against ORcTP. Col-0 seedlings were used as a negative control.
(E) BiFC observation showing that OR and TCP14 bind each other in the nucleus. C-terminal fragment of yellow fluorescent protein (cYFP) was used as a negative control. The YFP signal (green) and the signal from the nuclear marker VirD2NLS-mCherry (red) indicating the nucleus are shown. Bar = 10 μm. nYFP, N-termnial fragment of YFP.