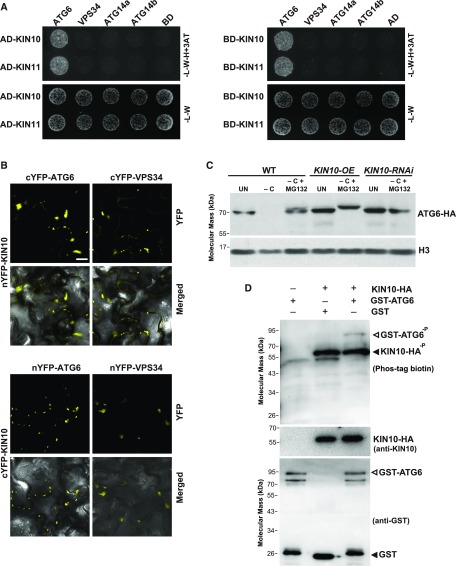
Figure 9.
SnRK1 Phosphorylates ATG6 during Fixed-Carbon Starvation.
(A) Y2H interactions of KIN10 or KIN11 with the subunits of the VPS34 complex. Full-length KIN10 or KIN11 expressed as N-terminal fusions to either the Gal4-activating (AD) or Gal4 DNA binding (BD) domains were coexpressed with complementary AD or BD fusions of ATG6, VPS34, ATG14a, and ATG14b on selection medium lacking Leu, Trp, and His and containing 10 μM 3-amino-1,2,4-triazole (-L-W-H+3AT; top panels), or on nonselection medium lacking Leu and Trp (-L-W; bottom panels).
(B) BiFC analysis testing the interaction of KIN10 with ATG6 and VPS34 in planta. N. benthamiana leaf epidermal cells were coinfiltrated with plasmids expressing the N- and C-terminal fragments of YFP fused to KIN10, ATG6, and VPS34. BiFC signals were detected by confocal fluorescence microscopy 36 h after infiltration. Bar = 10 μm.
(C) Prolonged fixed-carbon starvation and overexpression of KIN10 altered the SDS-PAGE mobility of ATG6. A plasmid encoding ATG6-HA was transiently expressed in protoplasts prepared from wild-type, KIN10-OE, or KIN10-RNAi plants for 16 h and then transferred to MS medium without Suc (−C) and incubated in darkness for 8 h with or without 50 μM MG132. Immunoblot analyses of protoplast extracts were performed with anti-HA antibodies. Immunoblot detection of histone H3 was used to confirm nearly equal protein loading. UN, protein samples extracted from protoplasts not exposed to fixed-carbon starvation.
(D) ATG6 is phosphorylated by KIN10 in vitro. Purified GST-ATG6 or GST was incubated with immunoprecipitated KIN10-HA in phosphorylation buffer followed by SDS-PAGE separation and immunoblotted with Phos-tag BTL-111, anti-KIN10, or anti-GST antibodies.