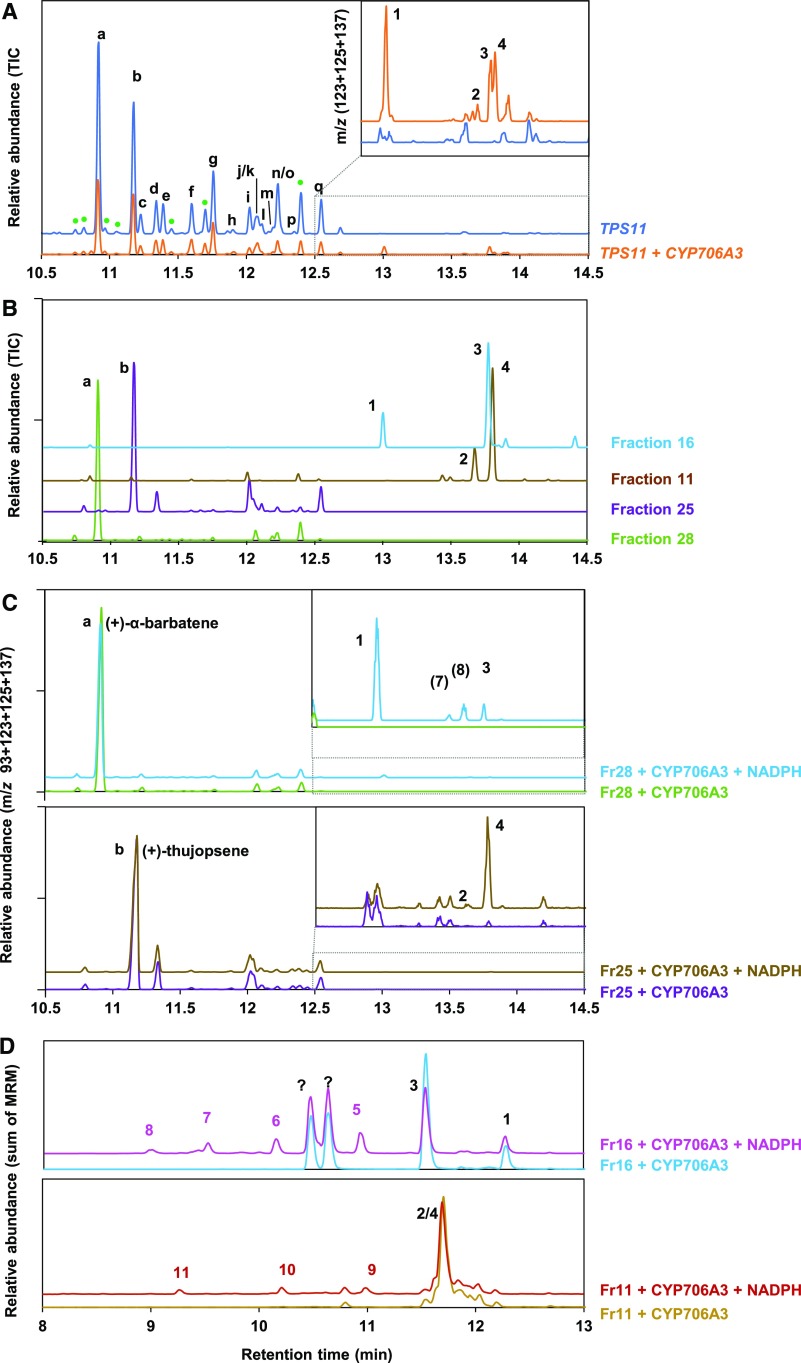
Figure 3.
Yeast-Expressed CYP706A3 Oxidizes TPS11 Products.
(A) GC-MS chromatograms of headspace collected from cultures of yeast expressing TPS11 or from mixtures of yeast expressing TPS11 or CYP706A3. Chromatograms show the relative abundance of total ion current (TIC) and the sum of extracted ion current as insets (m/z 123 + 125 + 137). See Methods for detailed protocol.
(B) GC-MS chromatograms of fractions purified from (A) and containing TPS11 and CYP706A3 products.
(C) GC-MS chromatograms of ethyl acetate extracts from incubations of purified TPS11 products [fraction 28 containing (+)-α-barbatene (top) or fraction 25 containing (+)-thujopsene (bottom)] with microsomal membranes prepared from yeast expressing CYP706A3 in the presence or absence (negative control) of NADPH. Chromatograms show the sum of extracted ion current (m/z 93 + 123 + 125 + 137). Insets show expanded chromatogram scale. CYP706A3-dependent metabolism of TPS11 products is probably underestimated due to low solubility of substrates in the incubation buffer.
(D) LC-MS/MS chromatograms of methanol extracts from incubations of purified CYP706A3 primary products [fraction 16 containing barbatene oxides (1) and (3; top) or fraction 11 containing thujopsene oxides (2) and (4; bottom)] with microsomal membranes prepared from yeast expressing CYP706A3 in the presence or absence (negative control) of NADPH. Chromatograms show the sum of different multiple reaction monitoring (MRM) listed in Supplemental Table 6.
TPS11 products are indicated with letters: (a) (+)-α-barbatene, (b) (+)-thujopsene, (c) isobazzanene, (d) (+)-β-barbatene, (e) (E)-β-farnesene, (f) β-acoradiene, (g) (+)-β-chamigrene, (h) α-zingiberene, (i) α-cuprenene, (j) α-chamigrene, (k) (-)-cuparene, (l) 1,2-dihydrocuparene, (m) (-)-zingiberene, (n) 1,2-dihydrocuparene, (o) β-sesquiphellandrene, (p) (E)-γ-bisabolene, and (q) δ-cuprenene. The products were identified based on a comparison of their MS and RT with libraries and published work (Tholl et al., 2005). CYP706A3 products are indicated by numbers and defined in Figure 4. Peaks labeled with question marks could not be identified, but their mass suggests that they are oxygenated sesquiterpenoids. These peaks appeared only after storage. See Supplemental Figures 3 and 4 for more extensive data.