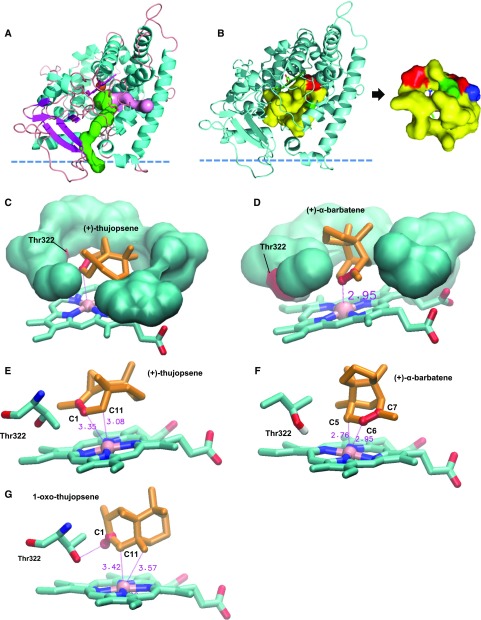
Figure 9.
3D Structural Model of the CYP706A3 Active Site and Comparative Docking of Relevant Substrates.
Structural model of AtCYP706A3 (segment 38-519) showing potential channels for substrate access, active site topology/characteristics, and docking positions for (+)-α-barbatene, (+)-thujopsene, and (+)-1-oxo-thujopsene determined by Autodock4.
(A) Overall structure displaying the two main channels (represented in surface mode in pink and green) with bottleneck values of 1.4 Å, positive hydropathy indexes, and logP values higher than 1. The predicted position of the membrane surface calculated by the PPM server of the OPM database (https://opm.phar.umich.edu/ppm_server) is schematized by the horizontal dashed line. The most hydrophobic channel (green) connects directly to the membrane bilayer while the other channel (pink) connects the active site to the interfacial medium.
(B) Display of the small internal cavity mostly delineated by hydrophobic residues (in yellow, surface mode). Two apertures are visible, corresponding to egress channels. Polar (green) and charged (red) residues correspond to Thr322 and Asp314, respectively. Thr322 is the only nonhydrophobic residue in contact with the active site cavity.
(C) View of (+)-thujopsene buried in the small hydrophobic pocket. Only the first crown of interacting residues is displayed. The only polar residue Thr322 of the cavity is shown on the left (red surface). The thujopsene double bond is indicated in red.
(D) View of (+)-α-barbatene in the small hydrophobic pocket. Only the first crown of residues is displayed. The only polar residue Thr322 of the cavity is shown on the left (red surface). The barbatene double bond is indicated in red. Distance (≈3Å) between the oxidation site and heme iron is indicated.
(E) Predominant docking pose of (+)-thujopsene, with high affinity energy binding of –9.2 kcal.mol–1. The two atoms closest to the heme iron are C1 and C11, in full agreement with oxidation site. Distances to heme iron are indicated in red with dotted lines, and double bond C1-C2 is shown in red.
(F) Predominant docking pose of (+)-α-barbatene, with high affinity energy binding of –9.39 kcal.mol–1. Two atoms (red ball-and-stick) closest to heme iron (pink) C5 and C6 are at equivalent distances. The polar side chain of Thr322 is displayed.
(G) Predominant docking pose of (+)-1-oxo-thujopsene, with high affinity energy binding of –9.8 kcal.mol–1. The keto group (C1=O), shown in red, is oriented on the same side as the polar side chain of Thr322.