Fig. 1.
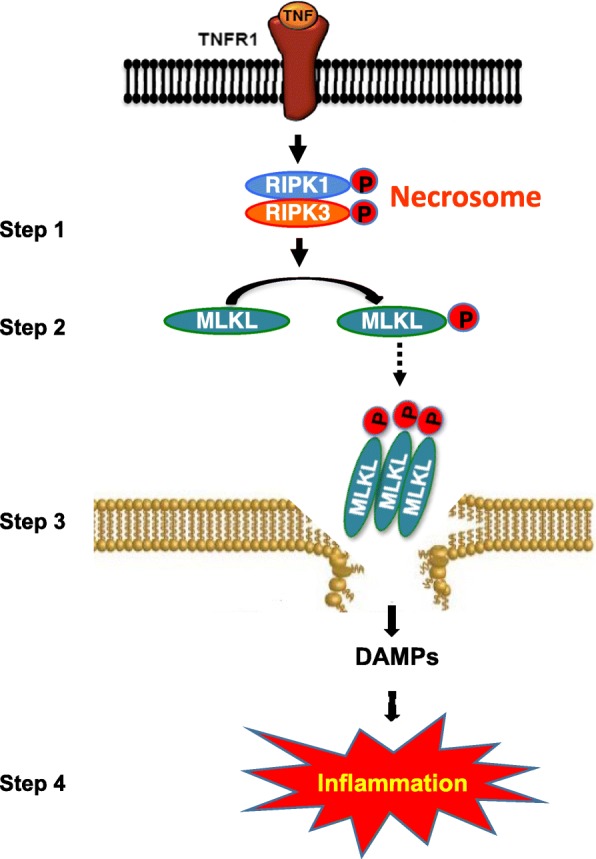
Schematic representation of TNF-α-induced necroptosis. Binding of TNF-α to its receptor, TNFR1, sequentially activates of RIPK1 and RIPK3 by phosphorylation, leading to the formation of necrosome, a complex of RIPK1 and RIPK3, which is a key event in necroptosis activation (step 1). This is followed by the phosphorylation of MLKL by active RIPK3, causing its oligomerization and membrane anchorage (step 2). Binding of oligomerized MLKL to the membrane causes its rapture and release of DAMPs (step 3). DAMPs bind to the cell surface receptors of innate immune cells, leading to increased transcription of proinflammatory cytokines and increased inflammation (step 4). RIPK, receptor-interacting protein kinase; MLKL, mixed lineage kinase domain-like protein
